DAN-222 from Dantari shows promise in reducing tumors in advanced HER2-negative breast cancer
Dantari, Inc. has released findings from their completed initial phase of clinical testing, which was an ascending dose trial. The results indicate that their compound, DAN-222, exhibits a favorable safety profile, is generally well received by the body, and has shown encouraging potential in reducing tumor development in individuals suffering from metastatic breast cancer that does not express human epidermal growth factor receptor 2 (HER2).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Investigation outcomes have indicated that 38% of subjects treated solely with DAN-222 experienced a period of disease stabilization, while a notable 67% of subjects treated with a regimen combining DAN-222 and a PARP inhibitor exhibited the same stabilization across varying dosage strengths. These findings are slated for disclosure through a visual presentation during today's poster session at the prestigious 2023 San Antonio Breast Cancer Symposium.
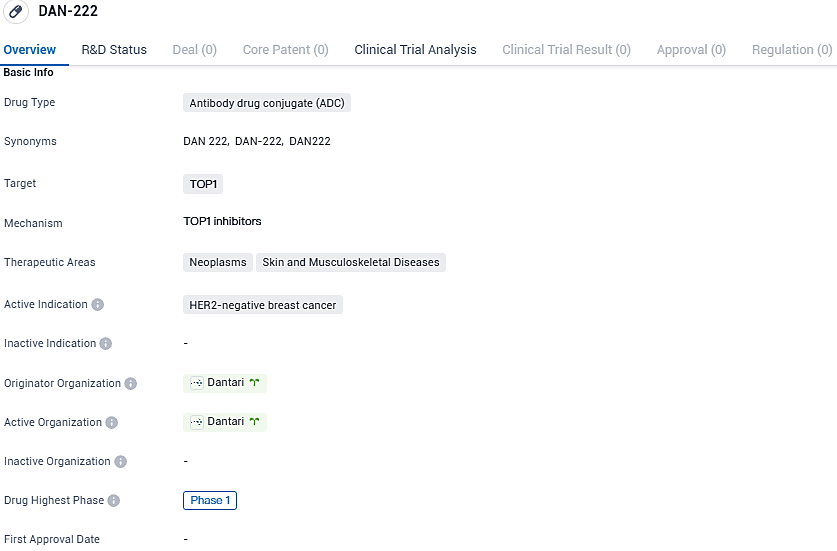
DAN-222 stands out as an innovative therapeutic candidate that pairs a topoisomerase 1 inhibitor with a drug conjugate known for its high drug delivery capacity. A primary concern associated with TOPO1 agents is the potential for damaging the bone marrow. However, DAN-222 showcases promising attributes, such as reduced bone marrow involvement in animal studies and a synergistic mode of action when used with PARP inhibitors. This positions DAN-222 as a versatile candidate suitable for wide-ranging clinical applications, offering a novel approach for combination treatment with PARP inhibitors and various other drugs. Notably, the enhanced therapeutic benefit appears to extend regardless of the BRCA mutation and across a spectrum of homologous recombination deficiencies.
Sara A. Hurvitz, M.D., FACP, the senior vice president and the head of the Clinical Research Division, expressed optimism regarding the preliminary results for DAN-222, highlighting the drug's safety and tolerability profile in patients with advanced breast cancer who had undergone extensive prior treatments. Dr. Hurvitz supports the further clinical investigation of DAN-222, including its potential synergy with PARP inhibitors.
Concurrently, Richard A. Markus, M.D., Ph.D., the president and chief executive officer of Dantari, reaffirmed the company's commitment to advancing DAN-222 into Phase 2 clinical testing. Dr. Markus emphasized that this progression aligns with their strategic goal of pioneering and providing innovative and effective high-capacity drug conjugate solutions to a diverse patient population in dire need of novel therapeutic avenues.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
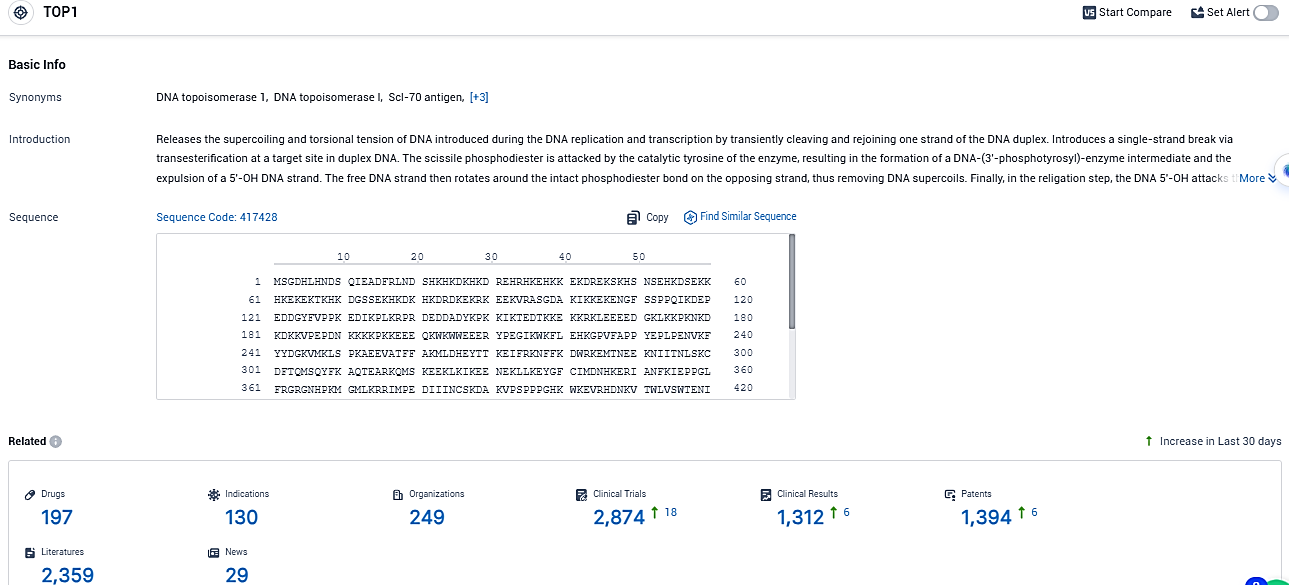
According to the data provided by the Synapse Database, As of December 15, 2023, there are 197 investigational drugs for the TOP1 target, including 130 indications, 249 R&D institutions involved, with related clinical trials reaching 2874, and as many as 1394 patents.
DAN-222 is an antibody drug conjugate being developed by Dantari for the treatment of HER2-negative breast cancer. It targets the TOP1 protein and falls within the therapeutic areas of neoplasms, skin, and musculoskeletal diseases. Currently in Phase 1 of development, DAN-222 holds promise as a targeted therapy that may improve treatment outcomes for patients with HER2-negative breast cancer.