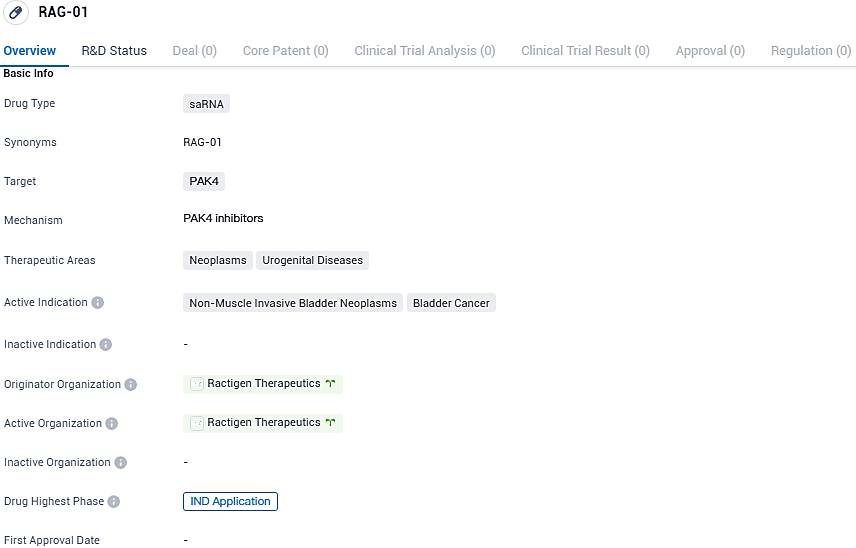
Ractigen Therapeutics Progresses to Clinical Trials with Innovative saRNA Medication, RAG-01
Ractigen Therapeutics, a frontrunner in small activating RNA (saRNA) therapeutic development, is pleased to disclose the lodgment of a clinical trial application within Australia. This submission lays the groundwork for initiating a phase I, open-label, multicentric clinical investigation focused on assessing the safety profile, tolerability, pharmacokinetic behaviors, and initial effectiveness of its pioneering saRNA pharmaceutical, RAG-01, specifically in individuals diagnosed with non-muscle-invasive bladder cancer who have shown resistance to previous Bacillus Calmette-Guérin treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Globally acknowledged as the second saRNA therapeutic to begin human trials, RAG-01 sets a precedent as the foremost in China to reach this crucial stage. This progress denotes a substantial leap in the field of RNA-focused medical treatments, with a noteworthy impact on the difficult arena of oncologic care.
Commenting on this pivotal achievement, Dr. Long-Cheng Li, the founder and guiding force behind Ractigen Therapeutics, conveyed his optimistic perspective: "To us, this milestone transcends a mere formality; it embodies a vital leap forward in utilizing RNAa methodologies in a clinical environment. Our own RNAa framework has been exemplified by the creation of RAG-01, which has demonstrated hopeful efficacy in preclinical oncology models and a robust safety record.
We are steadfast in our conviction of RAG-01's capability to propel the management of bladder cancer forward, and we are intent on continuing our mission to introduce more groundbreaking saRNA entities into clinical trials, aiming at illnesses that have notoriously been resistant to intervention.”
RAG-01 emerges as an innovative saRNA agent developed by Ractigen Therapeutics, formulated to specifically target and stimulate the p21 tumor suppressor gene. Administered via intravesical route with the state-of-the-art LiCO™ platform by Ractigen, the agent has exhibited pronounced effectiveness in diminishing tumor presence in murine orthotopic models of bladder cancer. Its advancement signifies a significant leap in the quest for RNA-driven therapeutic solutions, aiming to cater to the previously unaddressed medical needs of NMIBC patients.
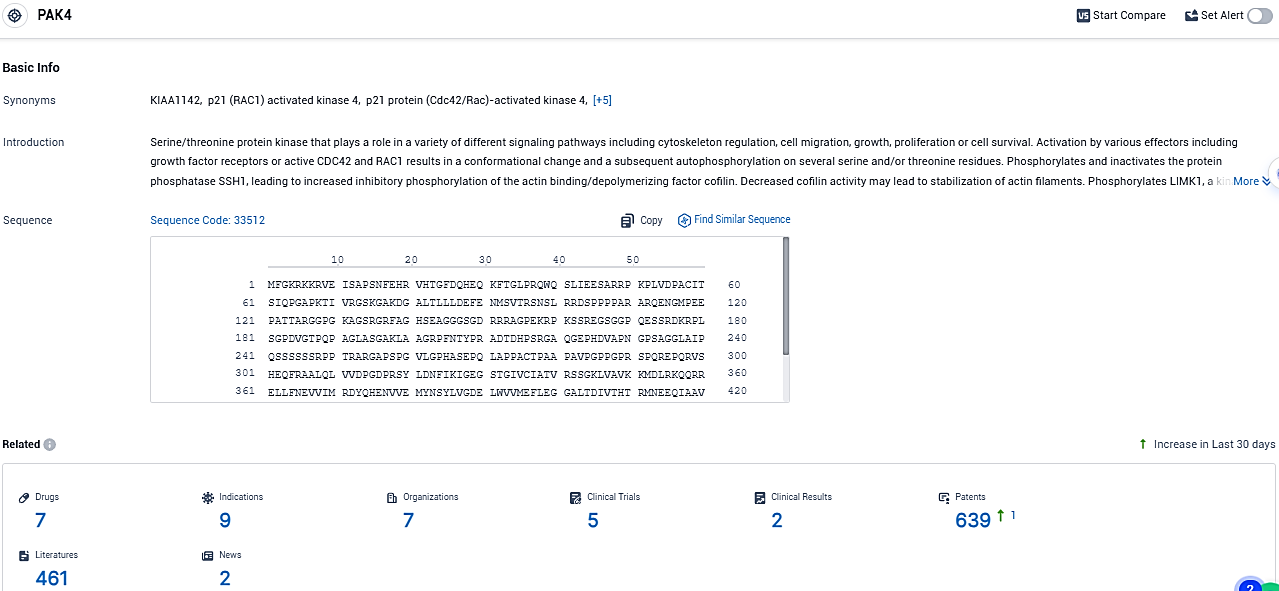
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 15, 2023, there are 7 investigational drugs for the PAK4 target, including 9 indications, 7 R&D institutions involved, with related clinical trials reaching 5, and as many as 639 patents.
RAG-01 shows potential as a treatment for non-muscle invasive bladder neoplasms, specifically bladder cancer. The targeting of PAK4 with saRNA technology suggests a novel approach to addressing this disease. However, further research and clinical trials are needed to determine the drug's safety and efficacy in humans.