Decoding Bromocriptine Mesylate: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Bromocriptine Mesylate's R&D Progress
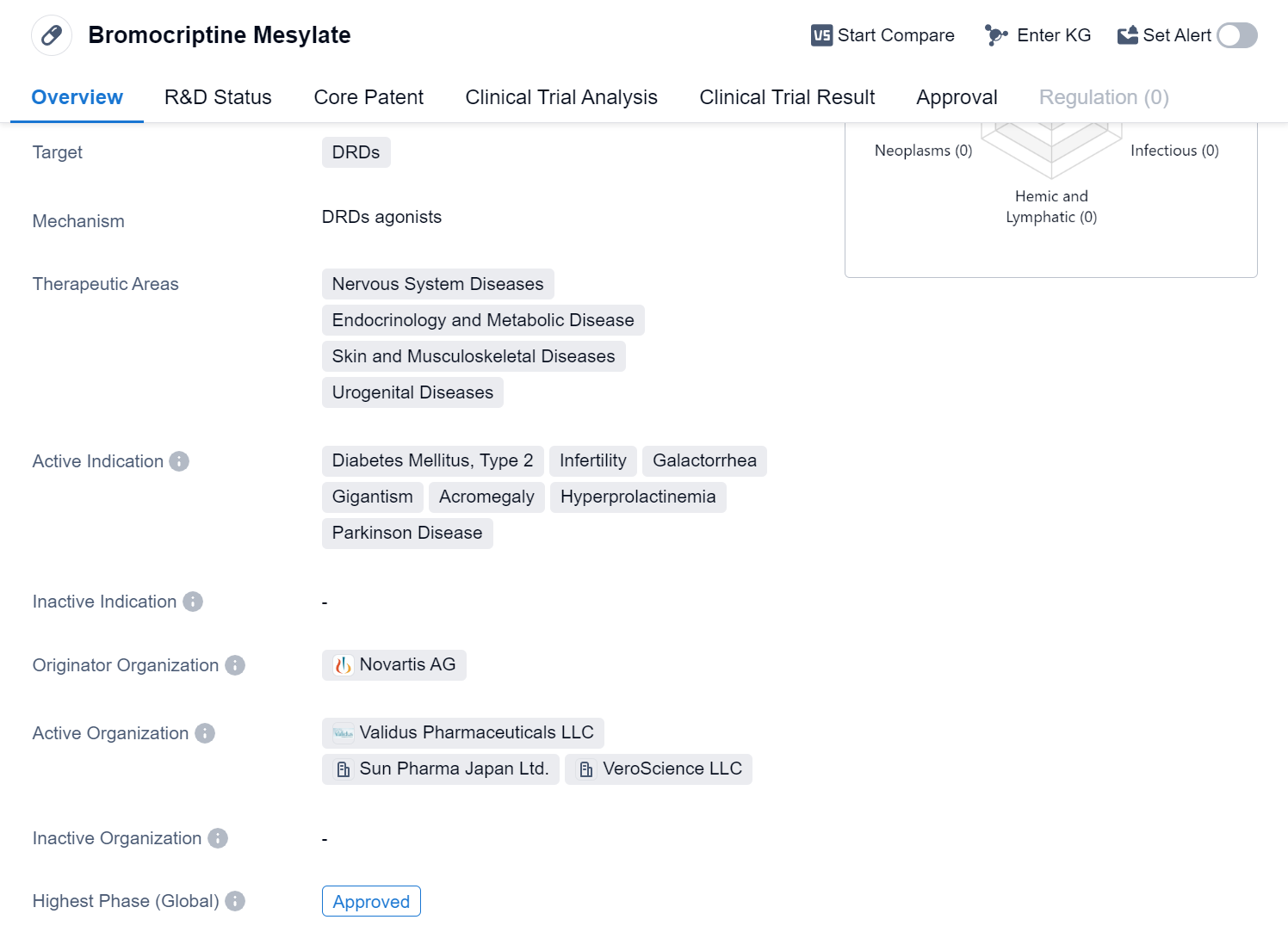
Bromocriptine Mesylate is a small molecule drug that primarily targets dopamine receptors (DRDs). It is used in the treatment of various diseases related to the nervous system, endocrinology and metabolic disorders, skin and musculoskeletal diseases, and urogenital diseases. The drug has been approved for several indications, including Diabetes Mellitus Type 2, infertility, galactorrhea, gigantism, acromegaly, hyperprolactinemia, and Parkinson's disease.
The originator organization of Bromocriptine Mesylate is Novartis AG, a multinational pharmaceutical company. The drug has achieved approval in global market, indicating its widespread acceptance and recognition. The first approval of Bromocriptine Mesylate took place in the United States in June 1978, making it a well-established medication with a long history of use.
Bromocriptine Mesylate's primary therapeutic application is in the management of Diabetes Mellitus Type 2. It helps regulate blood sugar levels by stimulating dopamine receptors in the brain, which in turn inhibits the release of insulin from the pancreas. By reducing insulin secretion, the drug helps control blood glucose levels and improves glycemic control in patients with type 2 diabetes.
Additionally, Bromocriptine Mesylate is used in the treatment of infertility, particularly in cases where elevated levels of prolactin are causing reproductive issues. It can also effectively manage galactorrhea, a condition characterized by the spontaneous production of breast milk in individuals who are not breastfeeding. The drug's ability to inhibit prolactin secretion helps alleviate these symptoms.
Furthermore, Bromocriptine Mesylate is employed in the management of gigantism and acromegaly, both of which are caused by excessive growth hormone production. By targeting dopamine receptors, the drug suppresses the release of growth hormone, thereby controlling the abnormal growth seen in these conditions.
Lastly, Bromocriptine Mesylate is utilized in the treatment of hyperprolactinemia, a condition characterized by elevated levels of prolactin hormone. The drug's ability to inhibit prolactin secretion helps normalize hormone levels and alleviate associated symptoms.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Bromocriptine Mesylate: DRDs agonists
From a biomedical perspective, DRDs agonists refer to drugs that activate dopamine receptors (DRDs) in the brain. Dopamine receptors are a type of G protein-coupled receptors that bind to the neurotransmitter dopamine. These agonists mimic the action of dopamine and stimulate the receptors, leading to various physiological and behavioral effects.
Dopamine receptors are classified into different subtypes, such as D1-like receptors (D1 and D5) and D2-like receptors (D2, D3, and D4). DRDs agonists can selectively target these receptor subtypes to produce specific therapeutic effects. For example, D2-like agonists are commonly used in the treatment of Parkinson's disease to alleviate motor symptoms.
By activating DRDs, agonists can modulate the dopamine signaling pathway and influence functions such as movement, reward, mood, and cognition. These drugs have diverse clinical applications, including the treatment of neurological and psychiatric disorders, such as Parkinson's disease, schizophrenia, and depression.
It's important to note that the specific effects and indications of DRDs agonists can vary depending on the subtype of dopamine receptor they target. Therefore, the term "DRDs agonists" encompasses a broad range of drugs with distinct pharmacological properties and therapeutic applications.
Drug Target R&D Trends for Bromocriptine Mesylate
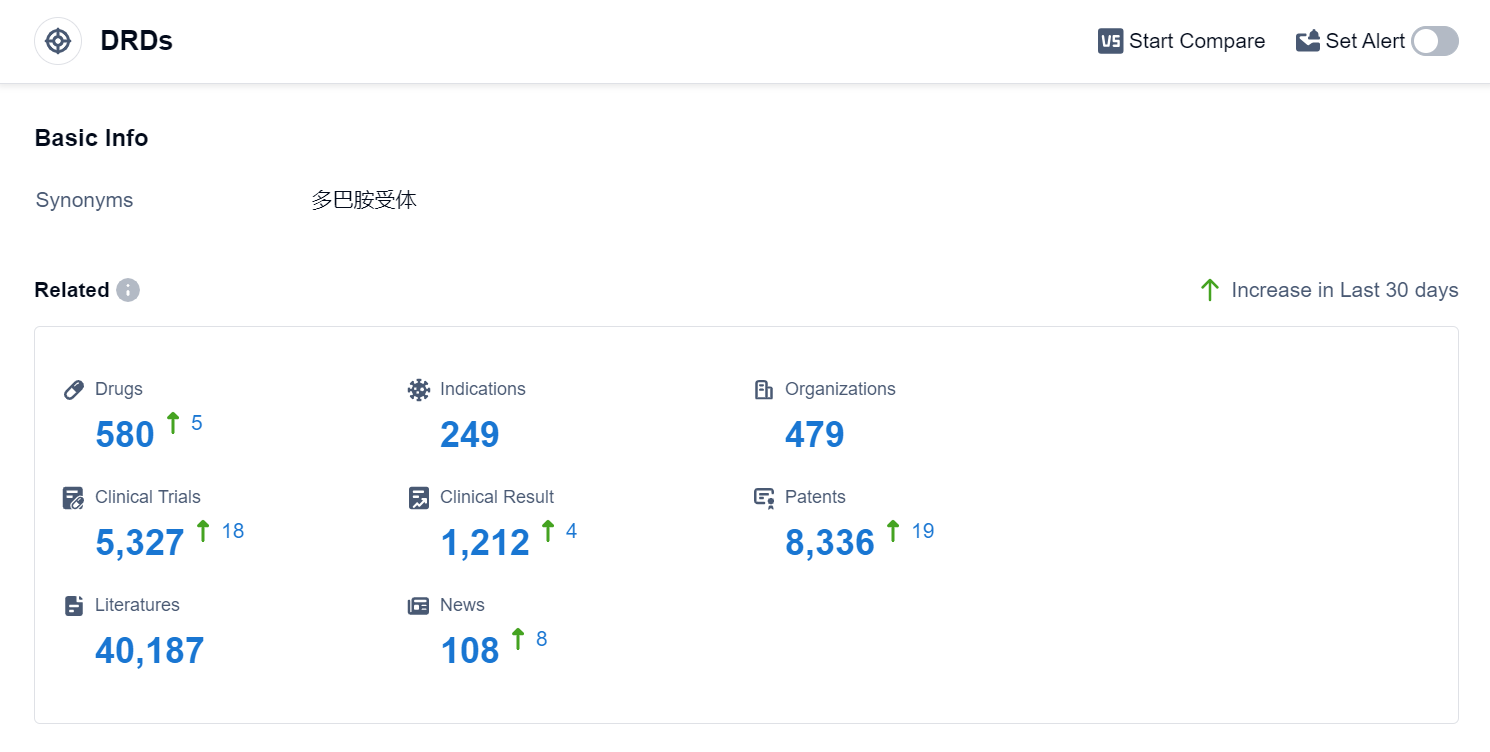
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 580 DRDs drugs worldwide, from 479 organizations, covering 249 indications, and conducting 5327 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target DRDs is characterized by the presence of several companies that are growing rapidly, with Johnson & Johnson, Novartis AG, and Mitsubishi Chemical Group Corp. leading the way. The highest stage of development is the approved stage, with drugs targeting indications such as Schizophrenia, Parkinson Disease, and Bipolar Disorder. China, the United States, and Japan are the countries/locations developing fastest under the current targets, with China showing significant progress. Overall, the target DRDs market is dynamic and competitive, with potential for further growth and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Bromocriptine Mesylate is a small molecule drug developed by Novartis AG. It targets dopamine receptors and is approved for various indications, including Diabetes Mellitus Type 2, infertility, galactorrhea, gigantism, acromegaly, hyperprolactinemia, and Parkinson's disease. Its first approval occurred in the United States in 1978, and it has since gained global acceptance. The drug's mechanism of action involves regulating dopamine receptors to achieve therapeutic effects in different disease areas.