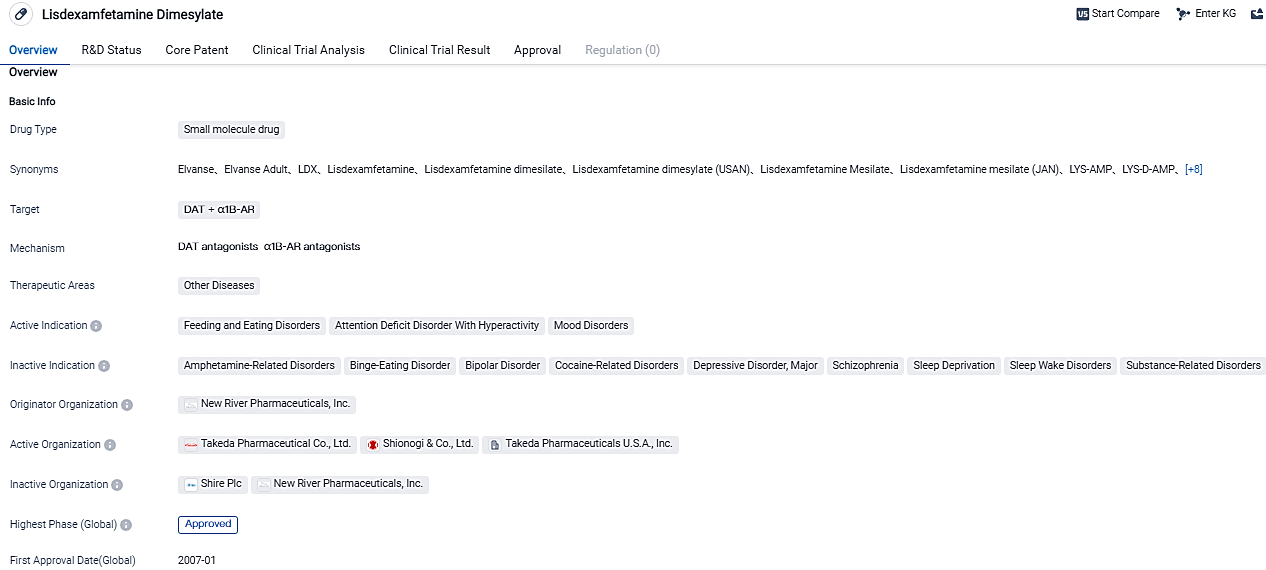
The FDA has given its approval to Mallinckrodt for marketing Dextroamphetamine for managing Attention Deficit/Hyperactivity Disorder (ADHD)
The FDA has given Mallinckrodt the green light to market Lisdexamfetamine Dimesylate Capsules, a product intended to mitigate Attention-Deficit/Hyperactivity Disorder (ADHD).
Mallinckrodt plc, a multinational specialty pharmaceutical firm, revealed today that its Specialty Generics division, under the business name SpecGx LLC, was granted approval by the United States Food and Drug Administration (FDA) on August 25, 2023, for its Abbreviated New Drug Application for Lisdexamfetamine Dimesylate Capsules 10mg, 20mg, 30mg, 40mg, 50mg, 60mg, and 70mg. The FDA declared the product by SpecGx LLC to be both bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Vyvanse® Capsules from Takeda Pharmaceuticals U.S.A., Inc., in every of the seven authorized strengths of the RLD.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Lisdexamfetamine Dimesylate capsules, a controlled substance recognized by the federal government, are employed to manage Attention-Deficit/Hyperactivity Disorder along with other conditions, and are presently listed in the FDA's drug shortage roster. The global revenue for Lisdexamfetamine Dimesylate surpassed $3.0 billion within Takeda's fiscal term that concluded on March 31, 2023.
Mallinckrodt initiated instant product commercialization after obtaining approval which was granted just a day subsequent to the expiration of the RLD's pediatric exclusivity. SpecGx LLC produces its generic version at its Hobart, New York station, using an active pharmaceutical ingredient fabricated in its St. Louis, Missouri facility.
Stephen Welch, the Executive Vice President and the Chief of Specialty Generics, stated, "Since Lisdexamfetamine Dimesylate finds its place in the current list of ADHD medications facing a shortage from the FDA, we are gratified to introduce this product at the moment and attempt to resolve the critical market need." He further added, "Our endeavor will be to closely coordinate with the Drug Enforcement Administration (DEA) to appeal and secure additional quota, facilitating an increase in our production post this approval, as we recognize the crucial role of affordable and superior quality generic ADHD drugs in patient access."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
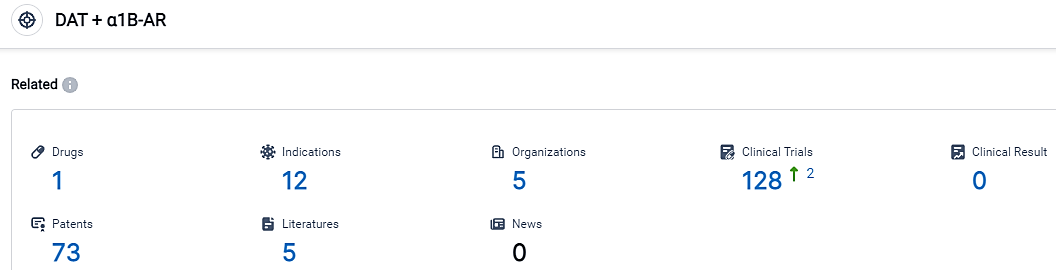
According to the data provided by the Synapse Database, As of September 2, 2023, 2023, there are 1 investigational drugs for the DAT and α1β-AR target, including 12 applicable indications, 5 R&D institutions involved, with related clinical trials reaching 128, and as many as 73 patents.
Mallinckrodt is an international corporation with numerous fully-owned subsidiary companies engaged in the development, production, marketing, and distribution of specialty pharmaceutical products and treatments. The firm's Specialty Brands identified section concentrates on areas such as neurology, rheumatology, hepatology, nephrology, pulmonology, ophthalmology, and oncology in autoimmune and rare diseases; neonatal respiratory critical care therapies and immunotherapy; analgesics; cultured skin alternatives, and gastrointestinal products. The Specialty Generics identified section of the company encompasses specialty generic medicines and active pharmaceutical ingredients.