Decoding Riluzole: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Riluzole's R&D Progress
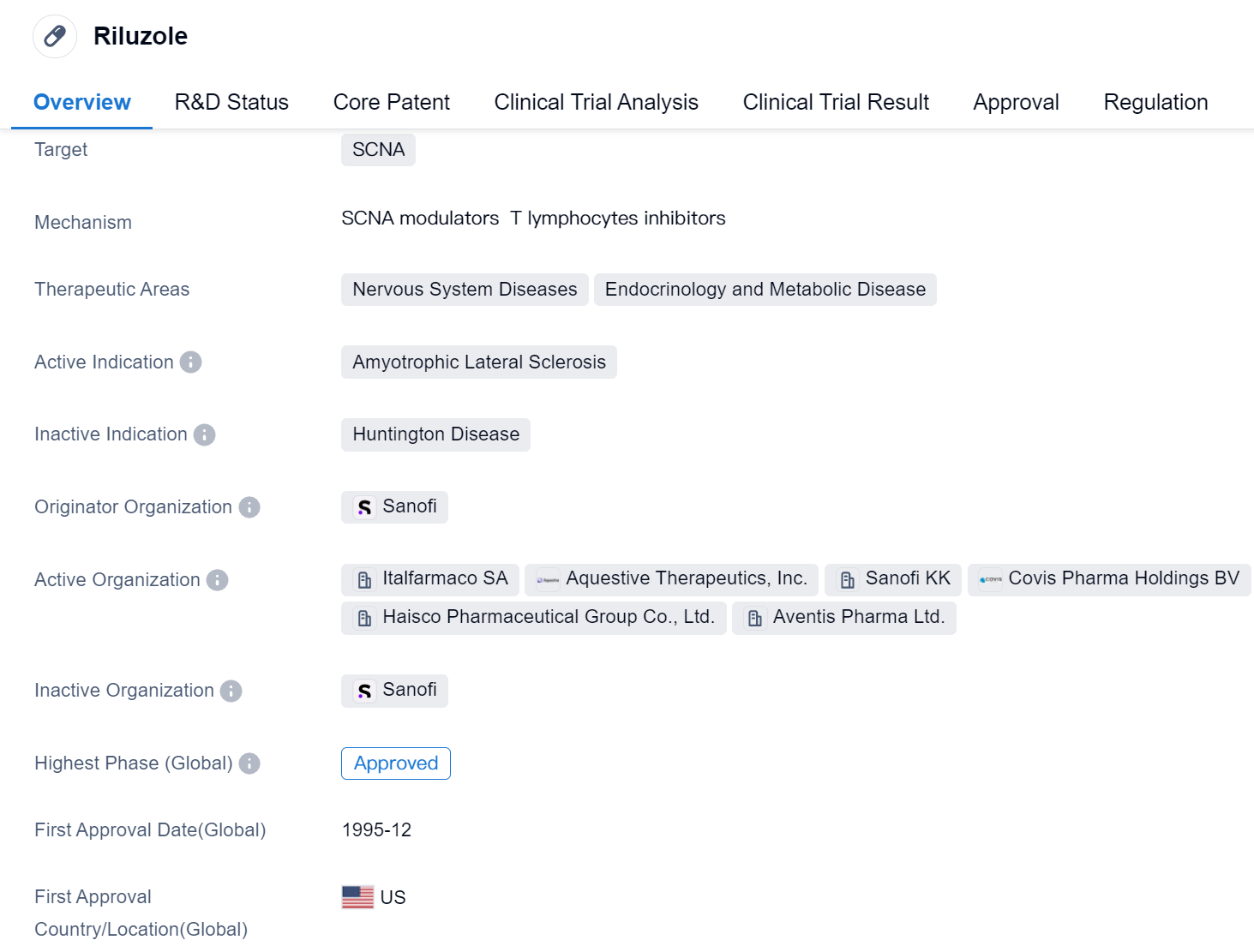
Riluzole is a small molecule drug that targets SCNA and is primarily used in the treatment of Amyotrophic Lateral Sclerosis (ALS). It falls under the therapeutic areas of Nervous System Diseases, Endocrinology, and Metabolic Disease. The drug was first approved in the United States in December 1995 and is regulated as an orphan drug.
Riluzole is developed by Sanofi, a renowned pharmaceutical company. It has achieved the highest phase of approval globally, indicating its effectiveness and safety in treating ALS.
As a small molecule drug, Riluzole is designed to target SCNA, which plays a crucial role in the nervous system. By targeting this specific protein, Riluzole aims to alleviate the symptoms and slow down the progression of ALS, a neurodegenerative disease that affects nerve cells in the brain and spinal cord.
The approval of Riluzole in 1995 marked a significant milestone in the treatment of ALS, providing patients with a viable option to manage their condition. Since then, it has been widely used globally, offering hope to individuals suffering from this debilitating disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Riluzole: SCNA modulators and T lymphocytes inhibitors
SCNA modulators: SCNA stands for Sodium Channel, Non-Selective, Activator. SCNA modulators are substances or drugs that can affect the activity of sodium channels in cells. Sodium channels play a crucial role in the generation and propagation of electrical signals in various tissues, including nerve cells and muscle cells. Modulating the activity of sodium channels can have significant effects on the function of these cells and the overall physiological processes they are involved in.
From a biomedical perspective, SCNA modulators can be used to regulate the excitability of cells, particularly in the nervous system. They can be used to treat conditions such as epilepsy, neuropathic pain, and cardiac arrhythmias, where abnormal sodium channel activity is implicated. By targeting and modulating sodium channels, these substances can help restore normal electrical signaling and improve the functioning of affected tissues.
T lymphocytes inhibitors: T lymphocytes, also known as T cells, are a type of white blood cell that plays a central role in the immune response. They are responsible for recognizing and eliminating foreign invaders, such as viruses and bacteria, as well as abnormal or cancerous cells in the body. However, in certain conditions, the immune system can become overactive and attack healthy tissues, leading to autoimmune diseases.
T lymphocyte inhibitors are drugs or substances that suppress or inhibit the activity of T cells. They can be used in the treatment of autoimmune diseases, organ transplantation, and certain types of cancer. By inhibiting T lymphocytes, these inhibitors help reduce the immune response and prevent the immune system from attacking healthy tissues.
In summary, SCNA modulators are substances that can affect the activity of sodium channels in cells, while T lymphocyte inhibitors are drugs that suppress the activity of T lymphocytes in the immune system. Both have important biomedical applications in the treatment of various conditions and diseases.
Drug Target R&D Trends for Riluzole
SCNAs, or Sodium Channel Navs, play a crucial role in the human body by facilitating the movement of sodium ions across cell membranes. These channels are responsible for generating and propagating electrical signals in various tissues, including the brain, heart, and muscles. By controlling the flow of sodium ions, SCNAs regulate the excitability and function of cells, enabling processes such as nerve transmission, muscle contraction, and cardiac rhythm. Dysregulation or mutations in SCNAs can lead to various disorders, including epilepsy, cardiac arrhythmias, and muscle disorders. Understanding the role of SCNAs is essential for developing targeted therapies and drugs to treat these conditions.
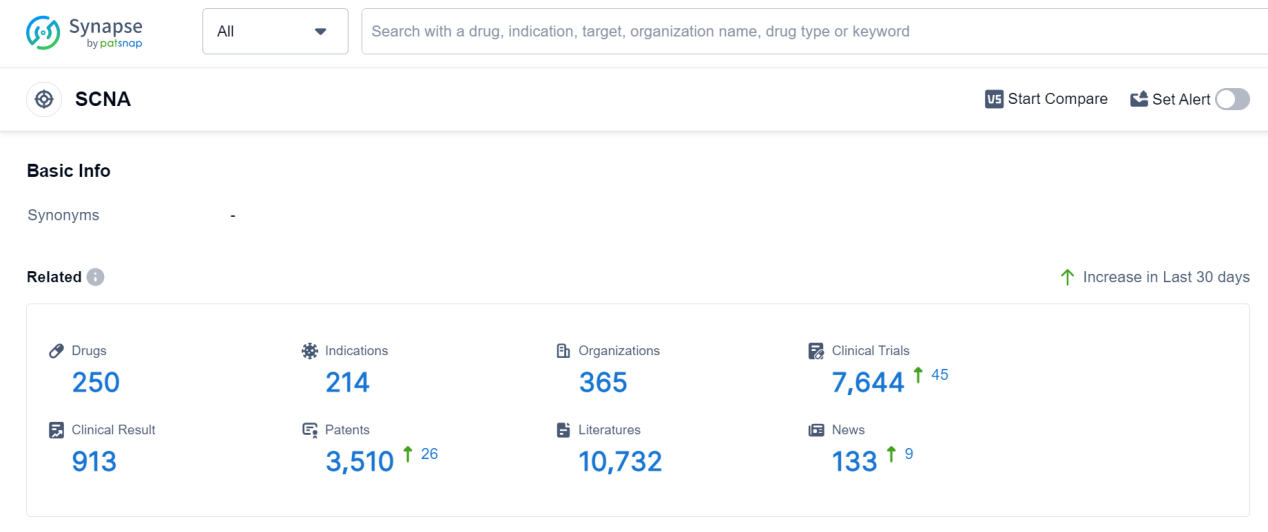
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 250 SCNA drugs worldwide, from 365 organizations, covering 214 indications, and conducting 7644 clinical trials
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Riluzole is a small molecule drug developed by Sanofi that targets SCNA and is primarily used in the treatment of Amyotrophic Lateral Sclerosis. It was first approved in the United States in 1995 and has achieved the highest phase of approval globally. As an orphan drug, Riluzole plays a crucial role in managing ALS and offers hope to patients suffering from this neurodegenerative disease.