Deep Scientific Insights on Clofibrate's R&D Progress, Mechanism of Action, and Drug Target
Clofibrate's R&D Progress
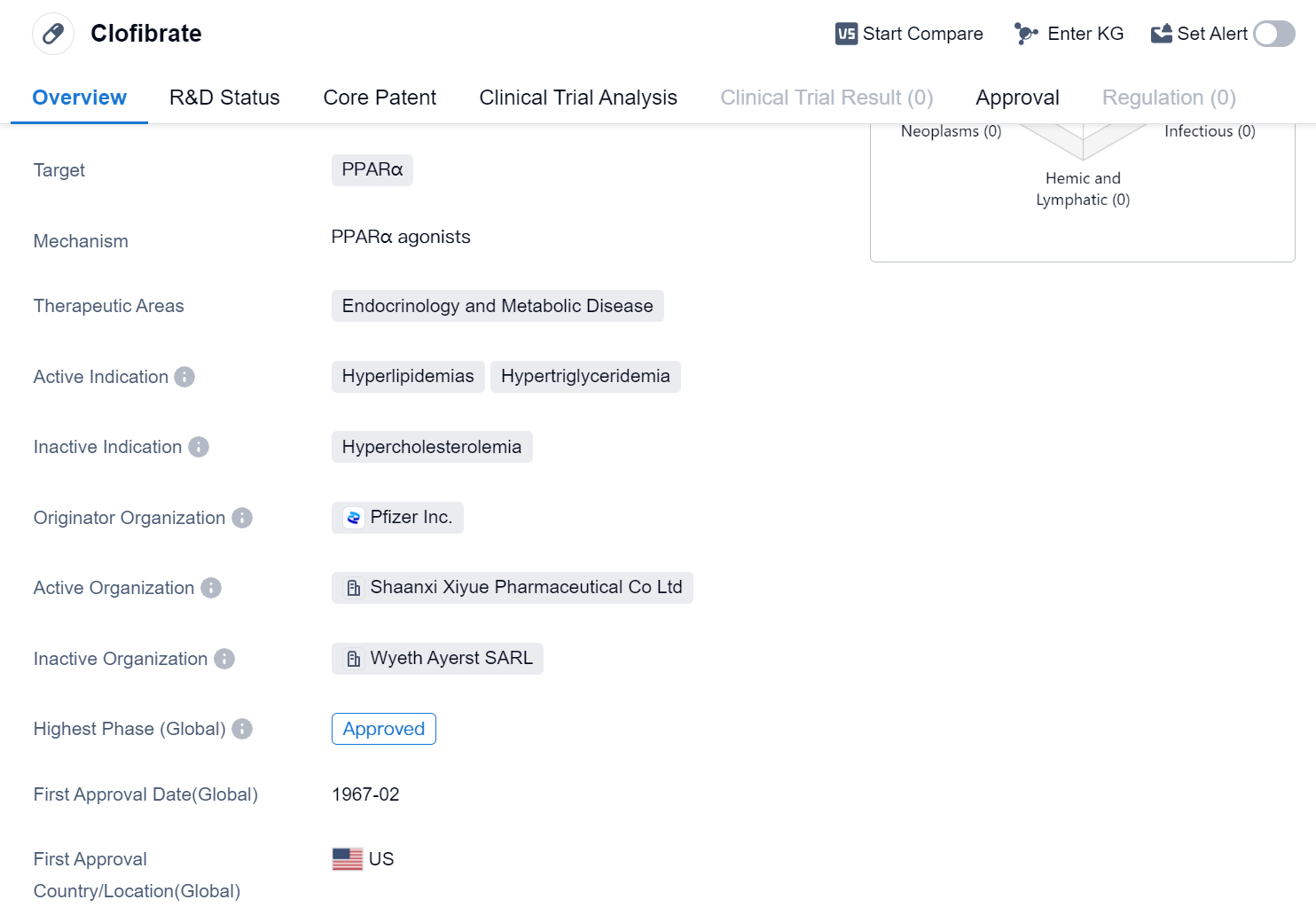
Clofibrate is a small molecule drug that falls under the therapeutic areas of endocrinology and metabolic disease. It primarily targets PPARα, a receptor involved in lipid metabolism. The drug is indicated for the treatment of hyperlipidemias and hypertriglyceridemia.
Clofibrate was developed by Pfizer Inc., a renowned pharmaceutical company. It has achieved approval in the global market, indicating its widespread acceptance and recognition. The drug received its first approval in the United States in February 1967, making it a well-established medication with a long history.
Hyperlipidemias refer to conditions characterized by elevated levels of lipids, such as cholesterol and triglycerides, in the blood. These conditions are often associated with an increased risk of cardiovascular diseases. Hypertriglyceridemia specifically refers to high levels of triglycerides, a type of fat, in the blood. Clofibrate works by activating PPARα, which regulates lipid metabolism and helps lower lipid levels in the body.
As a small molecule drug, Clofibrate is likely to have a relatively simple chemical structure, allowing for easy synthesis and formulation. This characteristic may contribute to its widespread use and availability in the market.
Its long history of use since 1967 further supports its established position in the treatment of hyperlipidemias and hypertriglyceridemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Clofibrate: PPARα agonists
PPARα agonists are a type of medication that activate the peroxisome proliferator-activated receptor alpha (PPARα) in the body. PPARα is a nuclear receptor that plays a crucial role in regulating lipid metabolism and inflammation. When PPARα is activated by agonists, it binds to specific DNA sequences and promotes the expression of genes involved in fatty acid oxidation and lipid metabolism.
From a biomedical perspective, PPARα agonists are used in the treatment of various metabolic disorders, particularly dyslipidemia (abnormal levels of lipids in the blood) and hypertriglyceridemia (high levels of triglycerides). These medications help to reduce triglyceride levels and increase high-density lipoprotein (HDL) cholesterol levels, thereby improving lipid profiles and reducing the risk of cardiovascular diseases.
Some examples of PPARα agonists include fenofibrate and gemfibrozil. These drugs are typically prescribed as part of a comprehensive treatment plan that includes lifestyle modifications such as a healthy diet and regular exercise. It is important to note that PPARα agonists may have side effects, including gastrointestinal disturbances and liver abnormalities, so they should be used under medical supervision.
In summary, PPARα agonists are medications that activate a specific receptor in the body to regulate lipid metabolism and reduce the risk of cardiovascular diseases. They are commonly used in the treatment of dyslipidemia and hypertriglyceridemia.
Drug Target R&D Trends for Clofibrate
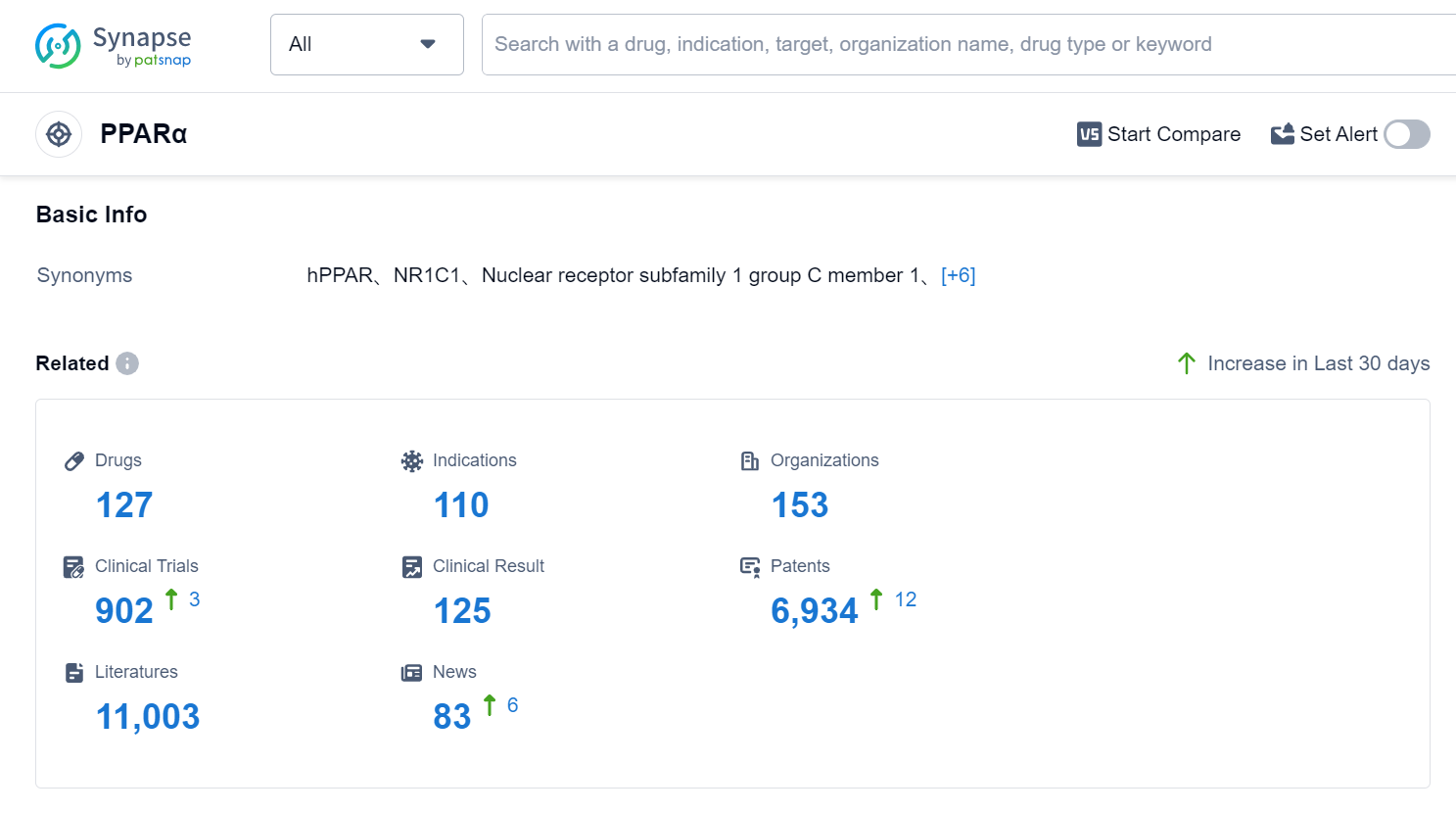
According to Patsnap Synapse, as of 1 Sep 2023, there are a total of 127 PPARα drugs worldwide, from 153 organizations, covering 110 indications, and conducting 902 clinical trials.
The analysis of the target PPARα reveals a competitive landscape with multiple companies actively involved in the development of drugs. Viatris Inc., Abbott Laboratories, Zydus Lifesciences Ltd., Kowa Co., Ltd., and Pfizer Inc. are some of the companies with significant R&D progress. The most approved indication for drugs targeting PPARα is hyperlipidemias, followed by hypertriglyceridemia, dyslipidemias, and other related indications. Small molecule drugs dominate the drug type analysis, with a high number of approved drugs. China is a leading country in the development of drugs targeting PPARα, with several approved drugs and ongoing research and development efforts. Overall, the target PPARα shows promise for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Clofibrate is a small molecule drug developed by Pfizer Inc. It targets PPARα and is indicated for the treatment of hyperlipidemias and hypertriglyceridemia. With approvals in the global market, Clofibrate has a long-standing presence in the pharmaceutical industry since its first approval in the United States in 1967.