Deep Scientific Insights on Nitazoxanide's R&D Progress
Nitazoxanide's R&D Progress
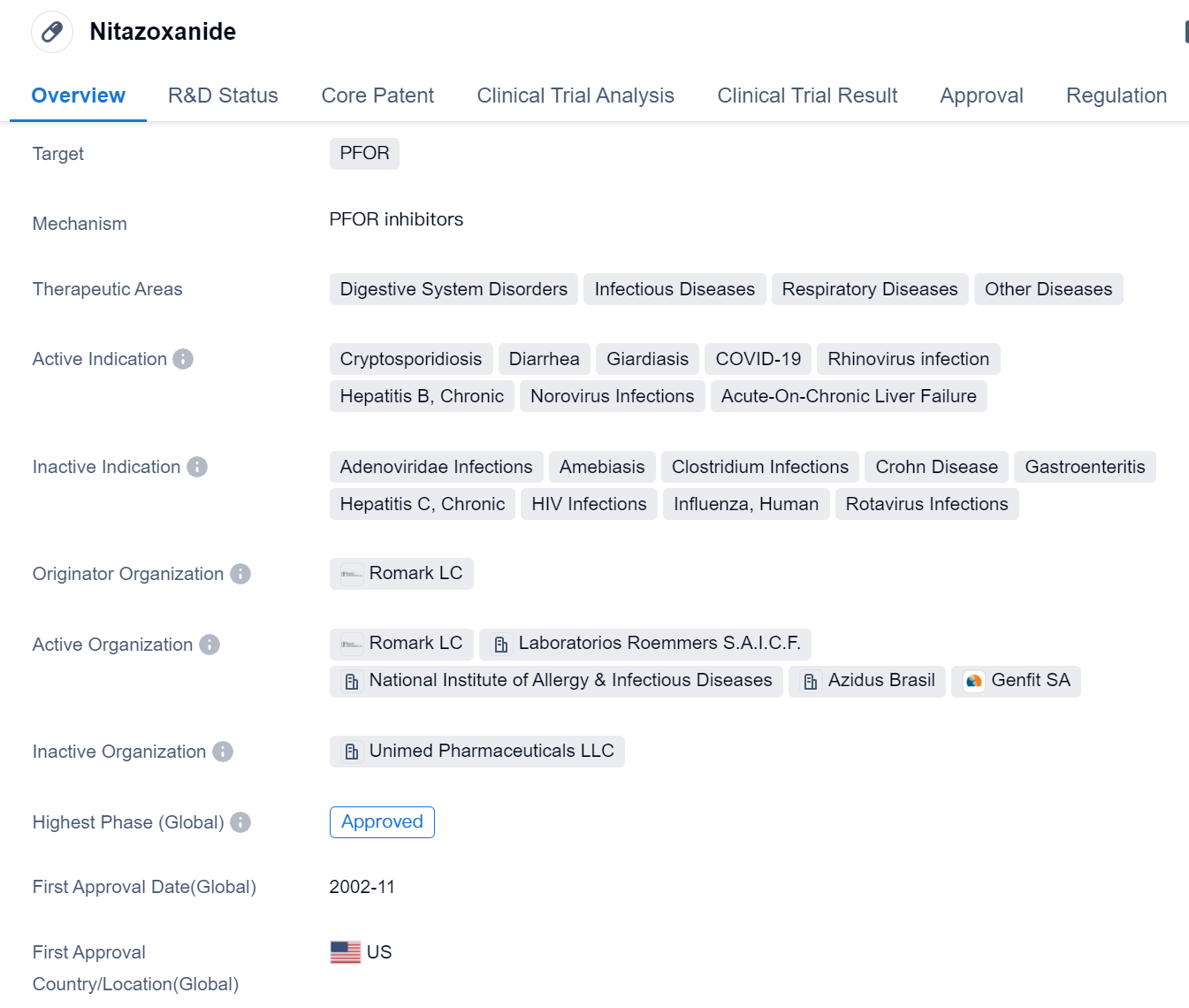
Nitazoxanide is a small molecule drug that primarily targets PFOR (pyruvate:ferredoxin oxidoreductase). It has been approved for use in various therapeutic areas, including digestive system disorders, infectious diseases, respiratory diseases, and other diseases. The drug has shown efficacy in treating several active indications, such as cryptosporidiosis, diarrhea, giardiasis, COVID-19, rhinovirus infection, hepatitis B (chronic), norovirus infections, and acute-on-chronic liver failure.
The originator organization of Nitazoxanide is Romark LC, and it received its first approval in the United States in November 2002. The drug is regulated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
Nitazoxanide has been widely used in the treatment of various infectious diseases, particularly those affecting the digestive and respiratory systems. It has shown effectiveness against parasites like Cryptosporidium and Giardia, which cause diarrhea and other gastrointestinal disorders. Additionally, Nitazoxanide has demonstrated antiviral activity against viruses like rhinovirus and norovirus, which are responsible for respiratory and gastrointestinal infections, respectively.
In recent years, Nitazoxanide has gained attention for its potential in treating COVID-19. Although it is not specifically approved for this indication, studies have shown that Nitazoxanide exhibits antiviral activity against SARS-CoV-2, the virus responsible for COVID-19. This has led to ongoing research and clinical trials to evaluate its efficacy in treating COVID-19 patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nitazoxanide: PFOR inhibitors
PFOR inhibitors are a type of drugs that target and inhibit the enzyme pyruvate:ferredoxin oxidoreductase (PFOR). PFOR is an important enzyme involved in the metabolism of certain microorganisms, particularly anaerobic bacteria and archaea. It plays a crucial role in the conversion of pyruvate to acetyl-CoA, which is a key step in the production of energy in these organisms.
By inhibiting PFOR, PFOR inhibitors disrupt the normal metabolic pathway and energy production in the targeted microorganisms. This can have therapeutic implications, especially in the treatment of infections caused by these microorganisms. PFOR inhibitors can potentially be used as antimicrobial agents to selectively target and kill anaerobic bacteria and archaea.
From a biomedical perspective, PFOR inhibitors hold promise in the development of new antibiotics or antimicrobial drugs. They offer a targeted approach to combat specific infections caused by anaerobic microorganisms, which can be challenging to treat with conventional antibiotics. Further research and development of PFOR inhibitors may lead to novel treatments for various infectious diseases.
Drug Target R&D Trends for Nitazoxanide
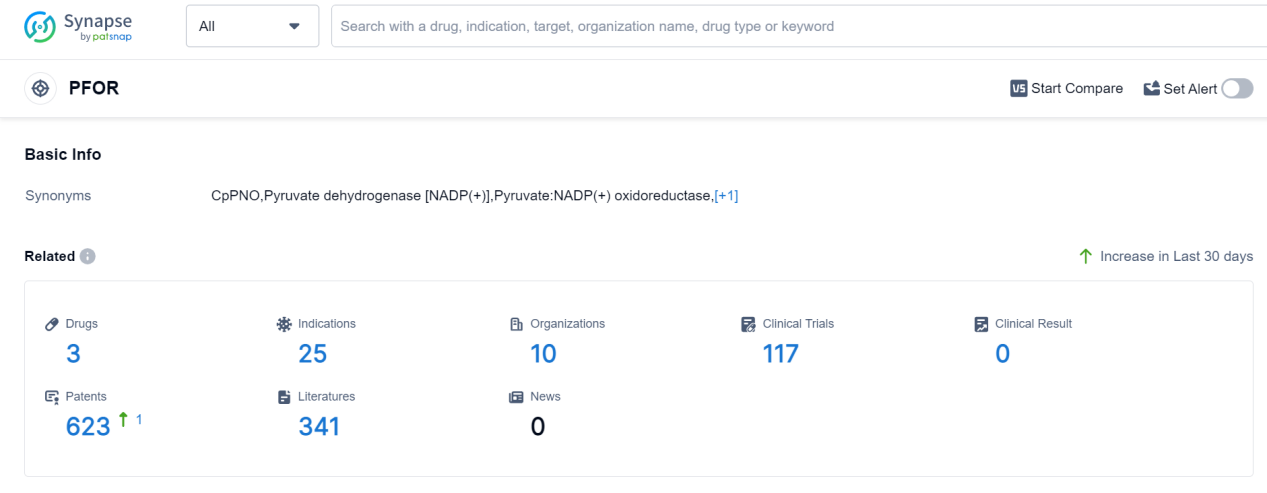
PFOR, or Pyruvate:ferredoxin oxidoreductase, plays a crucial role in the human body. It is an enzyme involved in the metabolic process of converting pyruvate, a product of glucose metabolism, into acetyl-CoA. This conversion is essential for the production of energy in the form of ATP. PFOR also participates in the fermentation process, allowing certain microorganisms to generate energy in the absence of oxygen. Additionally, PFOR is involved in the synthesis of important biomolecules, such as fatty acids and amino acids. Overall, PFOR is a key enzyme in various metabolic pathways, contributing to energy production and the synthesis of essential molecules in the human body.
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 3 PFOR drugs worldwide, from 10 organizations, covering 25 indications, and conducting 117 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Nitazoxanide is a versatile small molecule drug with a broad range of therapeutic applications. Its approval in multiple countries and its orphan drug status highlight its importance in addressing unmet medical needs. The drug's effectiveness against various infectious diseases, including COVID-19, makes it a valuable asset in the pharmaceutical industry. Ongoing research and development efforts will likely continue to explore its potential in treating other diseases and expanding its therapeutic applications.