Deep Scientific Insights on Rimonabant's R&D Progress, Mechanism of Action, and Drug Target
Rimonabant's R&D Progress
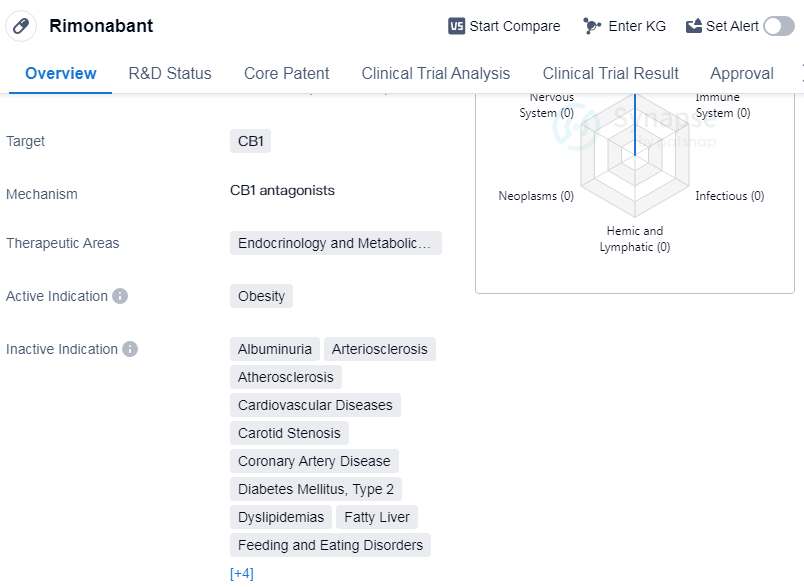
Rimonabant is a small molecule drug that targets the CB1 receptor and is primarily used in the therapeutic areas of endocrinology and metabolic disease. Its active indication is obesity. The drug was developed by Sanofi, a pharmaceutical company. Rimonabant has reached the highest phase of approval globally, and received its first approval in the European Union in June 2006.
Rimonabant's mechanism of action involves targeting the CB1 receptor, which is primarily found in the central nervous system. By blocking this receptor, the drug is believed to reduce appetite and food intake, making it a potential treatment option for obesity. However, it is important to note that the drug's development in China has been discontinued, suggesting that it may not have met the necessary requirements or faced challenges in the Chinese market.
The approval of Rimonabant in the European Union in 2006 marked an important milestone for the drug and its potential impact on the treatment of obesity.
As a small molecule drug, Rimonabant is likely to have undergone extensive research and development processes, including preclinical studies and clinical trials, to establish its safety and efficacy. The drug's approval in the European Union suggests that it has met the necessary regulatory requirements and demonstrated positive results in clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rimonabant: CB1 antagonists
CB1 antagonists are a type of drug that specifically target and block the CB1 receptors in the body. CB1 receptors are primarily found in the central nervous system, particularly in the brain, and are part of the endocannabinoid system. This system plays a role in various physiological processes such as pain perception, mood regulation, appetite, and memory.
CB1 antagonists work by binding to the CB1 receptors and preventing the activation of these receptors by endocannabinoids or other substances. By blocking CB1 receptors, these antagonists can modulate the effects of the endocannabinoid system. This can have therapeutic implications in various conditions and diseases.
From a biomedical perspective, CB1 antagonists have been studied for their potential use in the treatment of obesity, addiction, pain management, and neurological disorders.
It is important to note that CB1 antagonists can have side effects, as the endocannabinoid system is involved in many physiological processes. Additionally, the use of CB1 antagonists should be carefully monitored, as they may interact with other medications or have contraindications in certain medical conditions.
Overall, CB1 antagonists are a class of drugs that target the CB1 receptors in the body, offering potential therapeutic benefits in various biomedical applications.
Drug Target R&D Trends for Rimonabant
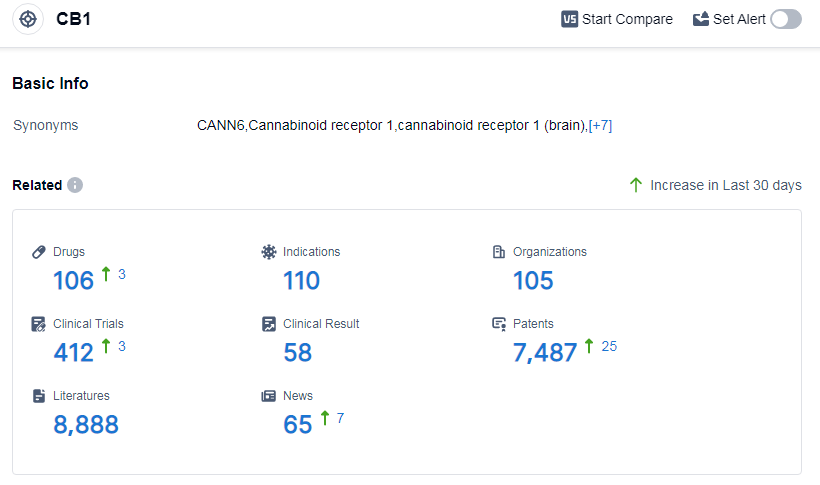
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 106 CB1 drugs worldwide, from 105 organizations, covering 110 indications, and conducting 412 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target CB1 in the pharmaceutical industry is characterized by the involvement of multiple companies at different stages of development. Sanofi is leading with one drug approved and one drug in phase 3. Indications such as obesity, neuralgia, cancer pain, and muscle spasticity have drugs approved under the target CB1. Small molecule drugs are progressing rapidly, indicating intense competition in the development of innovative drugs. The development of target CB1 is taking place in various countries/locations, including the European Union, Canada, Germany, Mexico, and others. Further analysis is required to assess the progress in China. Overall, the future development of target CB1 in the pharmaceutical industry shows potential for advancements in the treatment of various indications and the development of innovative drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Rimonabant is a small molecule drug developed by Sanofi that targets the CB1 receptor and is used in the therapeutic areas of endocrinology and metabolic disease. Its active indication is obesity, and it has received approval in the European Union. While its development in China has been discontinued, the drug's approval in the European Union highlights its potential as a treatment option for obesity, which is a significant global health concern.