Deep Scientific Insights on Tirbanibulin's R&D Progress, Mechanism of Action, and Drug Target
Tirbanibulin's R&D Progress
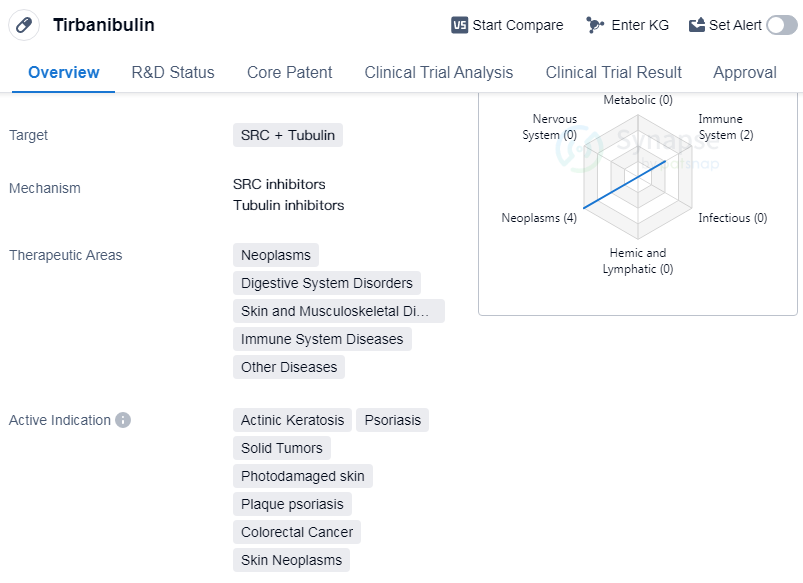
Tirbanibulin is a small molecule drug that targets SRC and Tubulin. It has shown potential therapeutic benefits in various therapeutic areas including neoplasms, digestive system disorders, skin and musculoskeletal diseases, immune system diseases, and other diseases. The drug has been indicated for the treatment of actinic keratosis, psoriasis, solid tumors, photodamaged skin, plaque psoriasis, etc.
Tirbanibulin was developed by Athenex, Inc., an originator organization in the pharmaceutical industry. It has reached the highest phase of development, which is approved globally. However, in China, it is still in the preclinical phase of development.
The drug received its first approval in December 2020 in the United States. This signifies that it has met the necessary regulatory requirements and has been deemed safe and effective for use in patients. The approval in the United States suggests that Tirbanibulin has undergone rigorous testing and evaluation to demonstrate its therapeutic benefits and safety profile.
Tirbanibulin's approval for multiple indications highlights its potential to address various medical conditions. Actinic keratosis, a common skin condition caused by sun damage, can be effectively treated with Tirbanibulin. Psoriasis, a chronic autoimmune disease affecting the skin, can also benefit from this drug. Additionally, Tirbanibulin shows promise in the treatment of solid tumors, colorectal cancer, and skin neoplasms, indicating its potential in combating cancerous growths.
The drug's mechanism of action, targeting SRC and Tubulin, suggests its ability to interfere with cancer cell growth and division. By targeting these specific proteins, Tirbanibulin may disrupt the processes that contribute to the development and progression of various diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tirbanibulin: SRC inhibitors and Tubulin inhibitors
SRC inhibitors are a type of drugs that specifically target and inhibit the activity of SRC enzymes. SRC is a protein kinase that plays a role in regulating cell growth, differentiation, and survival. By inhibiting SRC, these drugs can interfere with the signaling pathways involved in cancer development and progression.
Tubulin inhibitors, on the other hand, are drugs that target tubulin, a protein involved in the formation of microtubules. Microtubules are essential for cell division, and tubulin inhibitors disrupt their formation or stability, leading to cell cycle arrest and inhibition of cell proliferation. These drugs are commonly used in cancer chemotherapy to prevent the growth and spread of cancer cells.
In summary, SRC inhibitors specifically target SRC enzymes to interfere with cancer-related signaling pathways, while tubulin inhibitors disrupt the formation of microtubules, inhibiting cell division and proliferation.
Drug Target R&D Trends for Tirbanibulin
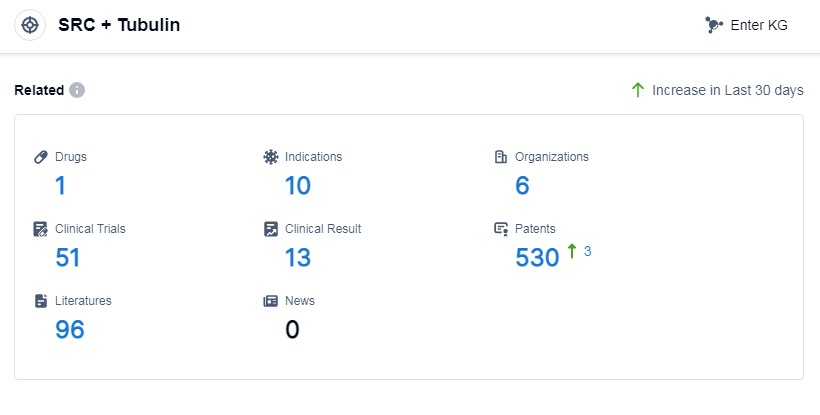
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 1 SRC and Tubulin drugs worldwide, from 6 organizations, covering 10 indications, and conducting 51 clinical trials.
The analysis of the current competitive landscape for the target SRC and Tubulin reveals that Grupo Corporativo Landon SL, PharmaEssentia Corp., Athenex, Inc., Fourth Military Medical University, and Xiangxue Pharmaceutical Co., Ltd. are the companies growing fastest under this target. The highest stage of development for this target includes approved, NDA/BLA, phase 2, phase 1/2, and preclinical. Drugs under this target have been approved for indications such as actinic keratosis, psoriasis, and solid tumors. Small molecule drugs are progressing most rapidly, indicating intense competition around innovative drugs. The countries/locations developing fastest under this target include the United States, Liechtenstein, European Union, United Kingdom, Norway, Switzerland, Hong Kong, Singapore, Malaysia, South Korea, Taiwan Province, China, and Macao. China has made progress in the preclinical stage of development.
Based on the analysis, the current competitive landscape for target SRC and Tubulin is diverse, with multiple companies and countries actively involved in research and development. The future development of this target is promising, with ongoing clinical trials and research efforts across various indications and drug types. Continued investment in R&D and strategic partnerships will be crucial for companies to stay competitive in this rapidly evolving pharmaceutical landscape.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Tirbanibulin is a small molecule drug developed by Athenex, Inc. that has shown potential in treating a range of medical conditions. Its approval in the United States and ongoing preclinical development in China indicate its progress in the pharmaceutical industry. Further research and clinical trials will be necessary to fully understand the drug's efficacy and safety in different patient populations.