Request Demo
Last update 03 Mar 2026

People's Liberation Army Air Force Military Medical University
Last update 03 Mar 2026
Overview
Tags
Neoplasms
Nervous System Diseases
Other Diseases
Small molecule drug
Monoclonal antibody
Recombinant polypeptide
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 26 |
| Monoclonal antibody | 6 |
| Recombinant polypeptide | 3 |
| circular RNA | 2 |
| Herbal medicine | 2 |
Related
66
Drugs associated with People's Liberation Army Air Force Military Medical UniversityTarget |
Mechanism SRC inhibitors [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date14 Dec 2020 |
Target |
Mechanism HDAC inhibitors [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. China |
First Approval Date23 Dec 2014 |
Target |
Mechanism BSG inhibitors |
Originator Org. |
Inactive Indication |
Drug Highest PhasePhase 3 |
First Approval Ctry. / Loc.- |
First Approval Date- |
283
Clinical Trials associated with People's Liberation Army Air Force Military Medical UniversityChiCTR2500104288
Research on the path of health behavior empowerment and key nursing techniques for elderly patients with chronic diseases
Start Date01 Apr 2025 |
Sponsor / Collaborator |
ChiCTR2500110636
Clinical study on paired associated magnetic stimulation therapy for post-stroke dysphagia
Start Date01 Apr 2025 |
Sponsor / Collaborator |
NCT06722534
Celecoxib for Prevention of Progression in Peutz-Jeghers Syndrome: A Double-blind, Randomized, Placebo-controlled Trial
The Peutz-Jeghers Syndrome (PJS) is a rare autosomal dominant syndrome characterized by mucocutaneous pigmentations, multiple gastrointestinal hamartomatous polyps, and an elevated risk of developing malignancies. Patients with PJS often experience recurrent gastrointestinal polyps that gradually increase in number and size, requiring repeated treatments. As the disease progresses, most patients are forced to undergo multiple surgical or endoscopic treatments. Small bowel polyps develop in 60-90% of patients with PJS, and intussusception occurs in 65% of these patients. Currently, on-demand surgery or scheduled endoscopic polypectomy is the standard of care for the management of small bowel polyps, and among patients who have undergone an initial surgery, reoperation is performed in up to 40% within 5 years. In addition, 8-40% of patients develop small bowel polyp-related complications even with multiple endoscopic treatments. However, surgery and endoscopic treatments are associated with complications, including short bowel syndrome, intestinal adhesions, bowel perforation and bleeding, and health-related quality of life. These problems often lead to decreased patient compliance and even treatment resistance, which increases the risk of disease progression. Because surgical and endoscopic treatment do not completely eliminate the potential for future polyps or extraintestinal neoplasms, there is an unmet medical need for the identification and use of pharmacologic agents to delay endoscopic or surgical interventions.
Cyclooxygenase (COX) is overexpressed in hamartomatous polyp tissue from PJS individuals, which may provide an avenue for possible effective chemoprevention of polyp formation and growth in PJS. Celecoxib, a COX-2 inhibitor, has been shown to reduce polyp burden by 54% in PJS model mice. In addition, the study evaluated the treatment effect of celecoxib on six patients with PJS, two of whom experienced a reduction in gastric polyp burden after six months. These findings provide preliminary evidence that celecoxib may delay the progression of PJS as a potential pharmacological prophylaxis.
Investigators plan to conduct a multicenter, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of celecoxib, and they will use a time-to-event analysis with a composite efficacy end point to determine whether celecoxib can delay disease progression or reduce the need for important endoscopic or surgical procedures in patients with PJS.
Cyclooxygenase (COX) is overexpressed in hamartomatous polyp tissue from PJS individuals, which may provide an avenue for possible effective chemoprevention of polyp formation and growth in PJS. Celecoxib, a COX-2 inhibitor, has been shown to reduce polyp burden by 54% in PJS model mice. In addition, the study evaluated the treatment effect of celecoxib on six patients with PJS, two of whom experienced a reduction in gastric polyp burden after six months. These findings provide preliminary evidence that celecoxib may delay the progression of PJS as a potential pharmacological prophylaxis.
Investigators plan to conduct a multicenter, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of celecoxib, and they will use a time-to-event analysis with a composite efficacy end point to determine whether celecoxib can delay disease progression or reduce the need for important endoscopic or surgical procedures in patients with PJS.
Start Date01 Feb 2025 |
Sponsor / Collaborator |
100 Clinical Results associated with People's Liberation Army Air Force Military Medical University
Login to view more data
0 Patents (Medical) associated with People's Liberation Army Air Force Military Medical University
Login to view more data
14,280
Literatures (Medical) associated with People's Liberation Army Air Force Military Medical University01 Mar 2026·Journal of the American Medical Directors Association
Prevalence and Risk Factors for Falls in Older Adults With Diabetes: A Systematic Review and Meta-Analysis
Review
Author: Lin, Yue ; Qi, Rui ; Chen, Xuan ; Yao, Jie ; Xiao, Yingjing ; Hua, Yan ; Liu, Tuonan ; Xu, Wenrong
OBJECTIVES:
To investigate the prevalence of falls and to assess risk factors associated with falls in older adults with diabetes.
DESIGN:
A systematic review and meta-analysis.
SETTING AND PARTICIPANTS:
Older adults with diabetes (≥60 years).
METHODS:
The literature search encompassed international (PubMed, Web of Science, Embase, Cochrane Library) and Chinese databases (CNKI, Wanfang, VIP, CBM) using systematic methods. The first search was conducted in June 2024, and the search was updated in May 2025. The 2 researchers independently conducted study selection, quality assessments, and data extraction. The meta-analysis was conducted using Stata 16.0 and RevMan 5.3. Pooled incidence rates and odds ratios for the prevalence of falls in older adults with diabetes, as well as for risk factors examined comparably in at least 2 studies, were calculated using fixed or random-effects models.
RESULTS:
The systematic review screened 5699 articles, ultimately analyzing data from 32 studies that included 23,666 older adults with diabetes. The pooled prevalence of falls in older adults with diabetes was 29.5%. This risk factor synthesis pooled data from 20 eligible studies, 15 distinct factors demonstrated statistically significant associations with falling incidents, including age, gender, timed up and go test, handgrip strength, cognitive dysfunction, depression, use of walking aids, gait issues, balance difficulties, weight loss, visual function abnormalities, diabetic retinopathy, hypoglycemia, diabetic peripheral neuropathy, and sleep quality.
CONCLUSIONS AND IMPLICATIONS:
Older adults with diabetes present a higher risk of falls. Health care providers should screen for factors associated with elevated fall risk and implement early interventions targeting modifiable risk factors to mitigate fall incidents in older adults with diabetes.
03 Feb 2026·PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA
Incomplete lineage sorting shaped mixed traits during a colobine primate radiation
Article
Author: Li, Bao-Guo ; Wu, Dong-Dong ; Zhang, Chi ; Li, Yingchun ; Huang, Zhipang ; Guo, Yan-Qing ; Garber, Paul A. ; Cui, Liangwei ; Li, Bei ; Wang, Yiming ; Zhang, Ru ; Qi, Xiao-Guang
Lineages undergoing rapid evolutionary diversification may evolve a set of mixed traits as a result of hybrid speciation, gene flow, or incomplete lineage sorting (ILS). However, how best to quantify these alternative processes impacting phenotypic variation remains unclear. Here, we examined trait evolution in two sister genera of endangered Asian primates,
Trachypithecus
and
Semnopithecus
. By assembling a de novo genome of the
Trachypithecus pileatus
group, which is geographically located in a transitional zone between these two genera, our integrated phylogenomic analyses clarified the evolutionary relationship of the
T. pileatus
group with other
Trachypithecus
species. We also identified that morphological similarities shared between this species group and
Semnopithecus
are the result of ILS, rather than gene flow or hybrid speciation. Across all chromosomes of the
T. pileatus
group, ILS contributed 8.9% of whole genome segments. Across these segments, we distinguished 77 genes such as
FGFBP1
and
FOXO1
that are involved in pathways of bone development and osteoblast differentiation. Functional experiments indicate that
FGFBP1
genotypes shared by species of the
T. pileatus
group and
Semnopithecus
appear to enhance osteogenic capability and mineralization, possibly resulting in larger body size and similarities in skull morphology compared with other species of
Trachypithecus
. The study reveals that ILS has shaped the evolution of mixed traits within gene-regulatory networks, driving phenotypic diversity during periods of rapid species divergence in this highly successful primate radiation. ILS appears to be more common than previously thought and represents a critical step in accurately assessing the phylogeny of closely related taxa.
01 Feb 2026·Advanced Science
Macrophagic Sclerostin Loop2‐ApoER2 Interaction Required by Sclerostin for Cardiovascular Protective Action
Article
Author: Shijian Ding ; Yuanyuan Yu ; Aiping Lu ; Ge Zhang ; Yu Huang ; Tao Zhang ; Shenghang Wang ; Hewen Jiang ; Sifan Yu ; Daqing Ma ; Zhanghao Li ; Xianghang Luo ; Shu Zhang ; Huarui Zhang ; Yihao Zhang ; Xiaoxin Wen ; Chuanxin Zhong ; Jin Liu ; Ning Zhang ; Baoting Zhang ; Luyao Wang ; Xin Yang ; Meiheng Sun ; Péter Ferdinandy ; Xiaohui Tao ; Xiaofei Li ; Nanxi Li ; Haitian Li
Abstract:
Therapeutic antibody against sclerostin loop2 promoted bone formation in postmenopausal osteoporosis but caused severe cardiovascular events in clinical applications. The studies of atherosclerosis and aortic aneurysm in
SOST
ki
.ApoE
−/−
mice and
sost
−/−
.
ApoE
−/−
mice collectively indicated the cardiovascular protective action of sclerostin. However, how sclerostin exerts cardiovascular protective action remains unclear. In this study, ApoER2 (LRP8) is notably identified as a novel transmembrane receptor for sclerostin in macrophages. Mechanistically, blockade of macrophagic sclerostin loop2‐ApoER2 interaction attenuates the suppressive effects of sclerostin on NF‐κB nuclear translocation, phosphorylation, and mRNA expression in macrophages, reduces the promotive effects of sclerostin on macrophage conversion to anti‐inflammatory phenotypes, and inhibits the preventive effects of sclerostin on atherosclerosis and aortic aneurysm in
ApoE
−/−
mice. Together, macrophagic sclerostin loop2‐ApoER2 interaction is required by sclerostin to suppress inflammatory responses, atherosclerosis, and aortic aneurysm in
ApoE
−/−
mice. Sclerostin plays a compensatory protective role in the cardiovascular system when
ApoE
is absent or mutated. Translationally, it provided critical pre‐clinical evidence regarding the prediction of cardiovascular risk populations (e.g.
, APOE
variants) for the marketed antibody against sclerostin loop2. Importantly, targeting sclerostin while preserving macrophagic sclerostin loop2‐ApoER2 interaction would offer the next generation of precise sclerostin inhibition strategy without cardiovascular safety concern, while promoting bone formation.
1
News (Medical) associated with People's Liberation Army Air Force Military Medical University05 Aug 2025
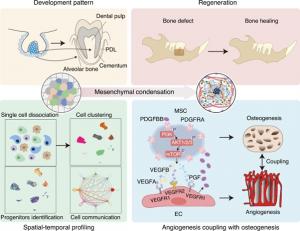
Single-cell transcriptomics reveal a distinct type of progenitor cell that supports angiogenesis and odontogenesis, leading to periodontal bone regeneration.
CHINA, August 5, 2025 /
EINPresswire.com
/ -- The precise mechanism of cellular condensation and regeneration is not well-understood in organogenesis. For advances in regenerative medicine, understanding these mechanisms is crucial. In a new study, researchers used single-cell transcriptomics to understand the composition of human dental follicles and dental papillae. They found a PDGFRA+ mesenchymal stem cell with odontogenic potential that interacts with endothelial cells via paracrine signaling to stimulate angiogenesis, showing promise for future therapeutics in dental regenerative medicine.
Stem cell research, alongside the rapidly advancing field of biotechnology, has led to remarkable innovations in regenerative medicine. The principles of organization and development of organ systems, generation of complex functionalities, and intricate tissue topography have proven useful in creating unique therapeutic approaches in regenerative medicine. In particular, researchers have been drawn to a phenomenon called “cell condensation/aggregation,” where stem cells such as mesenchymal stem/stromal cells (MSCs) possess an inherent ability to form compact cell assemblies. By exploiting this mechanism, researchers have shown that inducing aggregation of cultured MSCs can boost their regenerate organ structures such as teeth through epithelial-mesenchymal inductionive potential. This regenerative approach may help address challenging oral health problems, especially periodontal bone defects that cause significant bone damage and tissue loss.
During organogenesis, mesenchymal condensation helps create a signaling niche to recruit different interlineage progenitor cells to the specific region. For example, during tooth formation, the dental epithelium induces mesenchymal condensation in an environment that initially lacks vascularization. But the condensed mesenchyme then recruits endothelial progenitor cells (EPCs), which promote the assembly of vasculature in the tooth. However, the complex signaling mechanisms governing the interaction between MSCs and endothelial cells (ECs) that support tissue regeneration are not clearly understood.
Addressing this critical gap, a team of researchers including Dr. Fang Jin, Dr. Bingdong Sui, and Dr. Chenxi Zheng from The Fourth Military Medical University in China led a study to explore mesenchymal condensation-mediated tissue regeneration. They investigated the contribution of different stem cell types in the interlineage cell crosstalk in the context of tooth development and published their findings
in the International Journal of Oral Science
on 24 July 2025. Dr. Jin explains the methodological approach the team used for this study: “To tackle this complex issue, we employed single-cell RNA sequencing to explore the cellular composition and heterogeneity within the dental follicle and dental papilla developing tissues.” Using this technique, the team dissected the characteristic stem cell populations present in developing dental tissues in humans postnatally (after birth), such as dental follicle and dental papilla. Their results suggested that these two cell types have a common pool of stem cell populations.
Specifically, the stem cell populations known as dental follicle stem cells (DFSCs) and stem cells from apical papilla (SCAP) tissues exhibited similar molecular features, sharing 1,275 genes that were co-expressed in both stem cell types. To identify the genes that are specifically highly expressed in both these cell types to mark dental progenitor cells, the team performed a differential gene expression analysis and compared it with the rest of the cell populations. They found that platelet-derived growth factor receptor alpha (PDGFRA) was the only surface protein that was commonly expressed in these cell types, establishing the identity of MSCs. “As expected, PDGFRA showed expression specificity in DFSCs and SCAP and serves as a hallmark for common dental progenitor cells in DFSCs and SCAP in situ,” highlights Dr. Sui.
Further bioinformatic and biological assays demonstrated that ECs safeguard the functionality of PDGFRA+ MSCs via platelet-derived growth factor subunit BB (PDGFBB) and contribute to dental development. The PDGFRA+ MSCs in turn interact with CD31+ endomucin+ ECs via vascular endothelial growth factor A (VEGFA). This paracrine signaling mediates the formation of blood vessels during the development of periodontal development. Talking about the team’s findings in an in vivo donor-recipient bone regeneration experiment, Dr. Zheng explains, “Our in vivo experiments confirm that implanted PDGFRA+ cell aggregates persist in the recipient microenvironment, secrete factors aiding in angiogenesis, and potentially stimulate ECs to release PDGFBB for their own functional maintenance.” This communication between MSCs and ECs drives an active communication network that improves angiogenesis and osteogenesis and rapidly repairs the periodontal defects in their experiment.
Overall, the study reveals a specialized mesenchymal-endothelial crosstalk related to odontogenic condensation that could contribute to effective tissue regeneration, with potential applications in dental therapeutic strategies and even regenerative medicine in a broader context.
***
Reference
Title of original paper: Single-cell transcriptomics identifies PDGFRA+ progenitors orchestrating angiogenesis and periodontal tissue regeneration
Journal: International Journal of Oral Science
DOI:
10.1038/s41368-025-00384-6
Yini Bao
International Journal of Oral Science
+862885546461 ext.
ijos@scu.edu.cn
Visit us on social media:
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
100 Deals associated with People's Liberation Army Air Force Military Medical University
Login to view more data
100 Translational Medicine associated with People's Liberation Army Air Force Military Medical University
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 04 Mar 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Discovery
24
40
Preclinical
Phase 1
1
1
Phase 2
Other
7
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Meplazumab ( BSG ) | COVID-19 More | Phase 2 |
Metuzumab ( BSG ) | Non-Small Cell Lung Cancer More | Phase 1 |
Akkermansia muciniphila | Neuroinflammation More | Preclinical |
TriTE-N13 ( CD3 x CD80 x PSMA ) | Prostatic Cancer More | Preclinical |
Nuciferine ( D1 receptor x D2 receptor x TAS2R46 ) | Ischemic stroke More | Preclinical |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free




