Detalimogene Shows 71% Full Response Rate at Any Point in Early LEGEND Study Cohort Analysis
enGene Holdings Inc. (Nasdaq: ENGN), is advancing in the field of clinical-stage genetic medicines. Their primary non-viral investigational therapy, detalimogene voraplasmid (known as detalimogene or formerly EG-70), is currently undergoing a critical study involving patients diagnosed with high-risk, Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (Cis). Recently, enGene released early findings from the evaluation of 21 patients at the three-month mark, with 17 of these patients also assessed at six months, within the key cohort of the ongoing LEGEND study. The findings revealed a Complete Response (CR) rate at any time of 71%, a CR rate at three months of 67%, and a CR rate at six months of 47%. The treatment was mostly well-tolerated, and no patients ceased participation due to adverse events related to the therapy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“We are encouraged by this initial dataset, which unmistakably shows that detalimogene is both highly efficacious and generally well tolerated. These findings align with those from Phase 1 and bolster our confidence in its emerging profile,” stated Raj Pruthi, M.D., Chief Medical Officer of enGene. “Additionally, we are planning to refine the protocol in LEGEND, which we anticipate will offer patients further clinical benefits.”
“The early efficacy and safety outcomes observed in LEGEND’s pivotal cohort, along with detalimogene’s ease of handling, administration, minimal storage needs, and lack of post-procedural restrictions, highlight its potential to become a widely used treatment for NMIBC patients, particularly within community practice,” commented Suzanne Merrill, M.D., a bladder cancer specialist at the United Urology Group in Colorado.
Bladder cancer ranks among the top 10 cancers in terms of incidence in the US, with substantial annual treatment costs. NMIBC constitutes over 75% of bladder cancer cases, and more than 70% of urologists operate within community settings where most of these patients receive treatment.
“Detalimogene was engineered to be the most practical therapy for urologists managing NMIBC,” remarked Ron Cooper, Chief Executive Officer of enGene. “The preliminary results from our pivotal LEGEND study clearly show that detalimogene has the potential to offer a distinctly advantageous profile by achieving the optimal balance of efficacy, tolerability, and ease of use.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
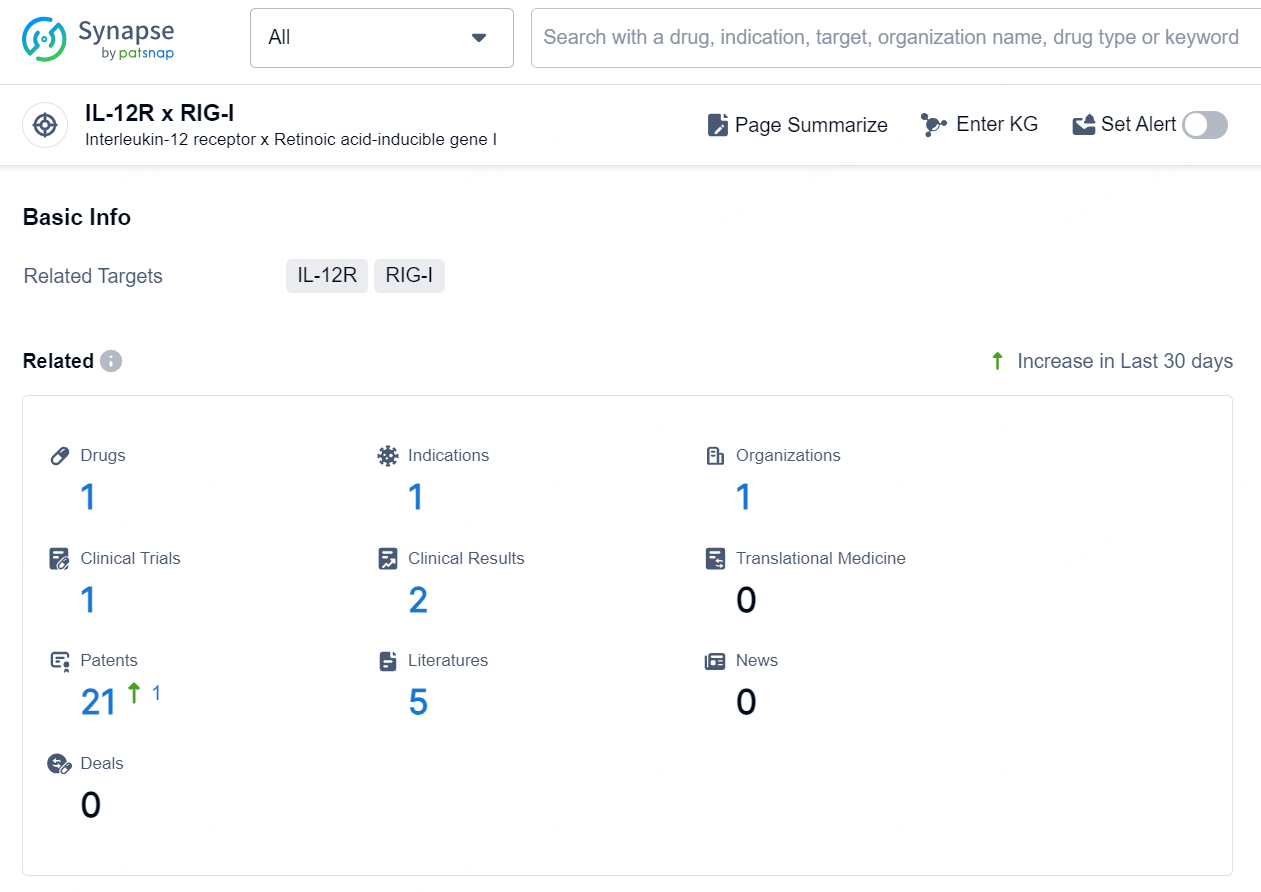
According to the data provided by the Synapse Chemical, As of September 30, 2024, there are 1 investigational drug for the IL-12R and RIG-I target, including 1 indication, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 21 patents.
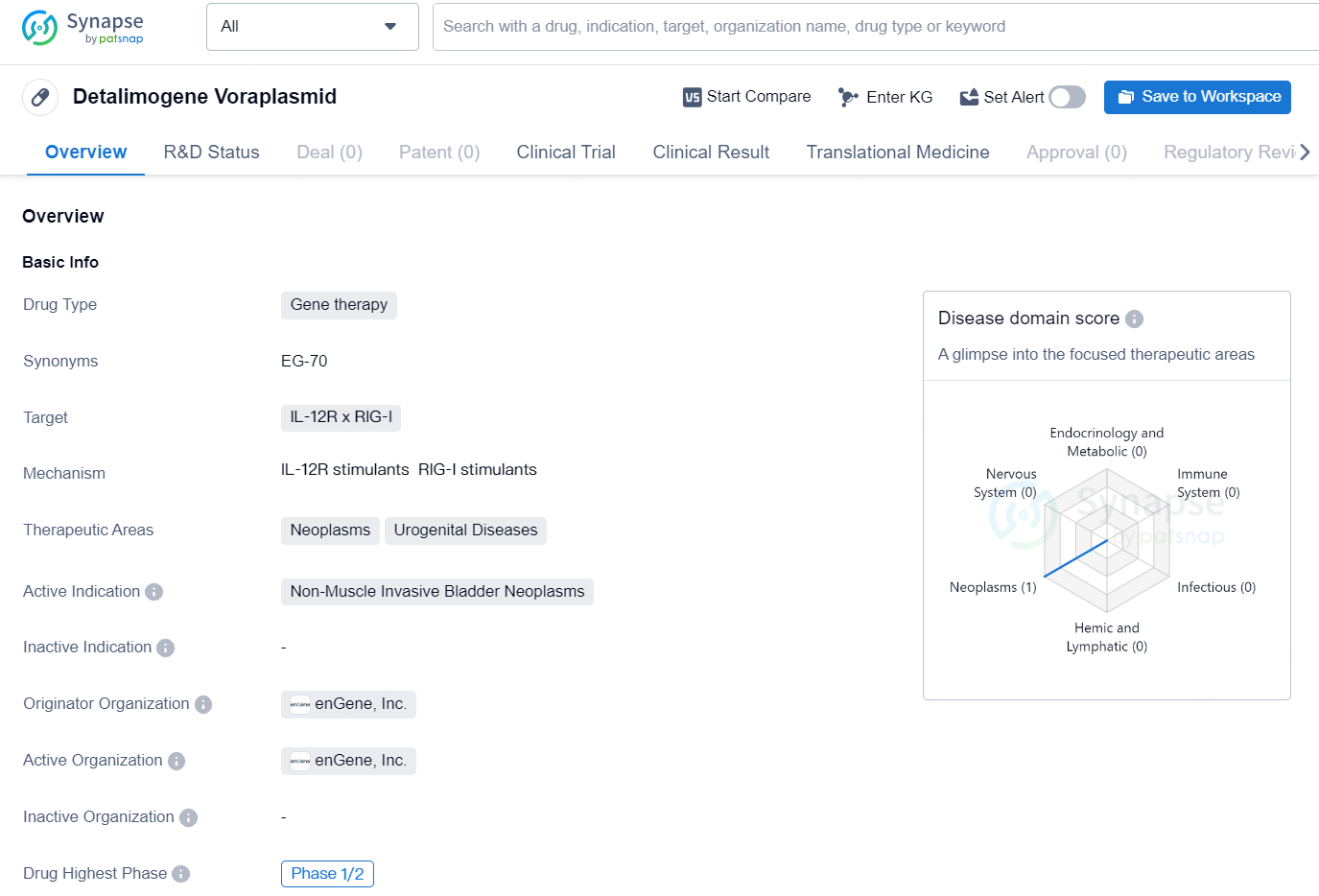
Detalimogene Voraplasmid is a gene therapy drug that targets IL-12R and RIG-I. It is primarily focused on treating neoplasms and urogenital diseases, with a specific active indication for non-muscle invasive bladder neoplasms. The drug is being developed by enGene, Inc., a pharmaceutical company that specializes in the development of nucleic acid-based therapeutics.