ReCode Therapeutics Begins Phase 1b Trial of RCT2100 for Cystic Fibrosis Treatment
ReCode Therapeutics, a company engaged in developing genetic medicines at the clinical stage, harnessing tissue-specific delivery to advance mRNA and gene correction therapies, revealed that they have administered the first dose to a patient in a Phase 1b clinical trial assessing RCT2100. This investigational inhaled mRNA treatment is targeted at individuals suffering from cystic fibrosis (CF).
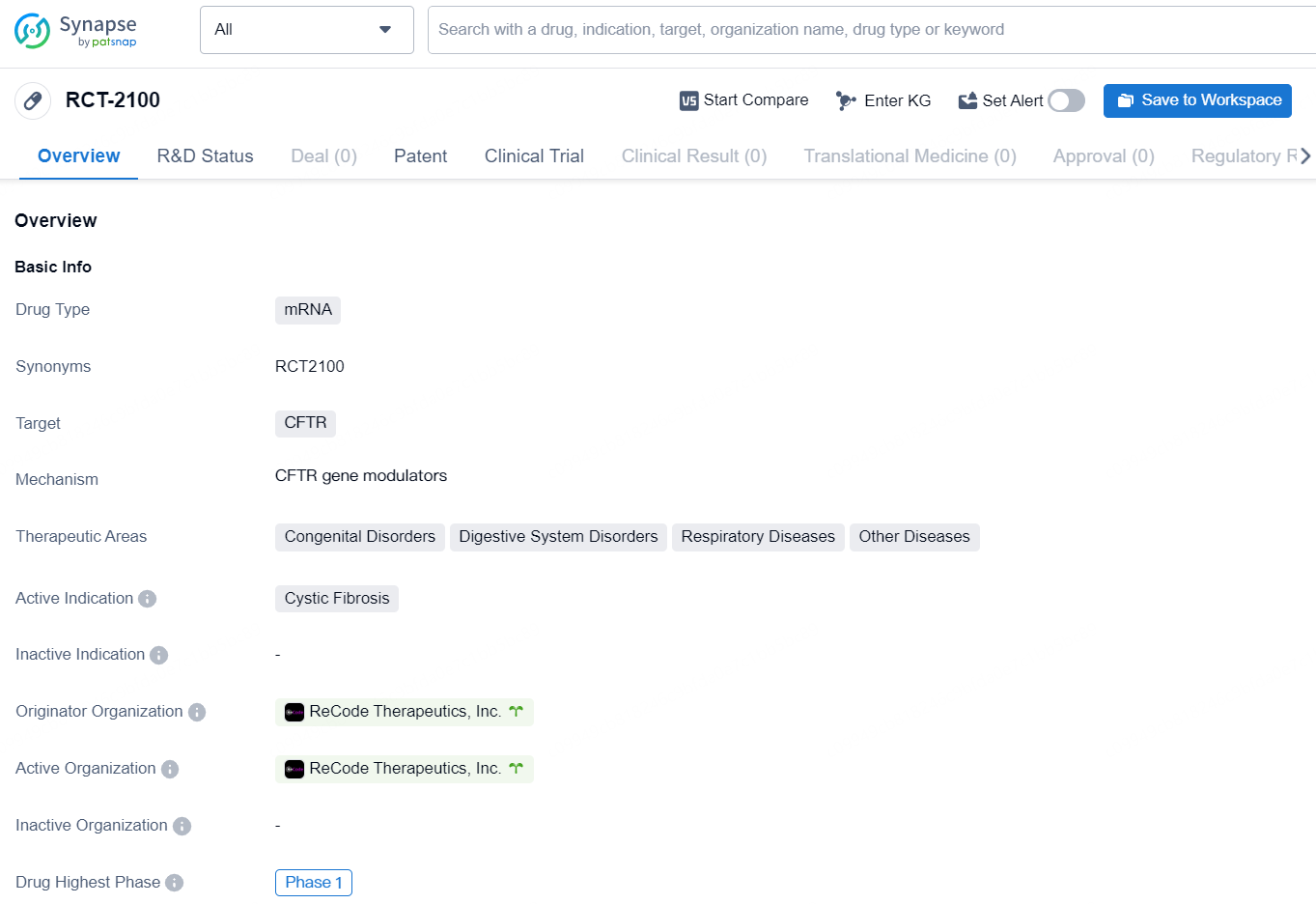
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
CF arises from alterations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, affecting roughly 130,000 individuals globally. Despite significant progress in CFTR modulator therapies, certain mutations result in an absence of functional CFTR protein, leaving these patients without effective treatment options.
“The commencement of our Phase 1b trial of RCT2100 in individuals with cystic fibrosis represents a crucial advancement and brings us closer to offering new treatment possibilities to those unserved by current CF therapies,” remarked Shehnaaz Suliman, M.D., MBA, M.Phil., CEO of ReCode Therapeutics. “We deeply appreciate the continuous support and collaboration of the CF patient community and are committed to expediting trial enrollment to deliver this potential new therapy to those in need as swiftly as possible.”
Developed using ReCode’s unique Selective Organ Targeting (SORT) lipid nanoparticle (LNP) system, RCT2100 is intended to transport CFTR mRNA directly to lung cells, prompting them to synthesize a functional CFTR protein absent in some CF patients. By supplying this CFTR mRNA, the therapy aims to tackle the fundamental cause of CF, potentially reinstating proper CFTR protein function in the lungs rather than merely alleviating the symptoms.
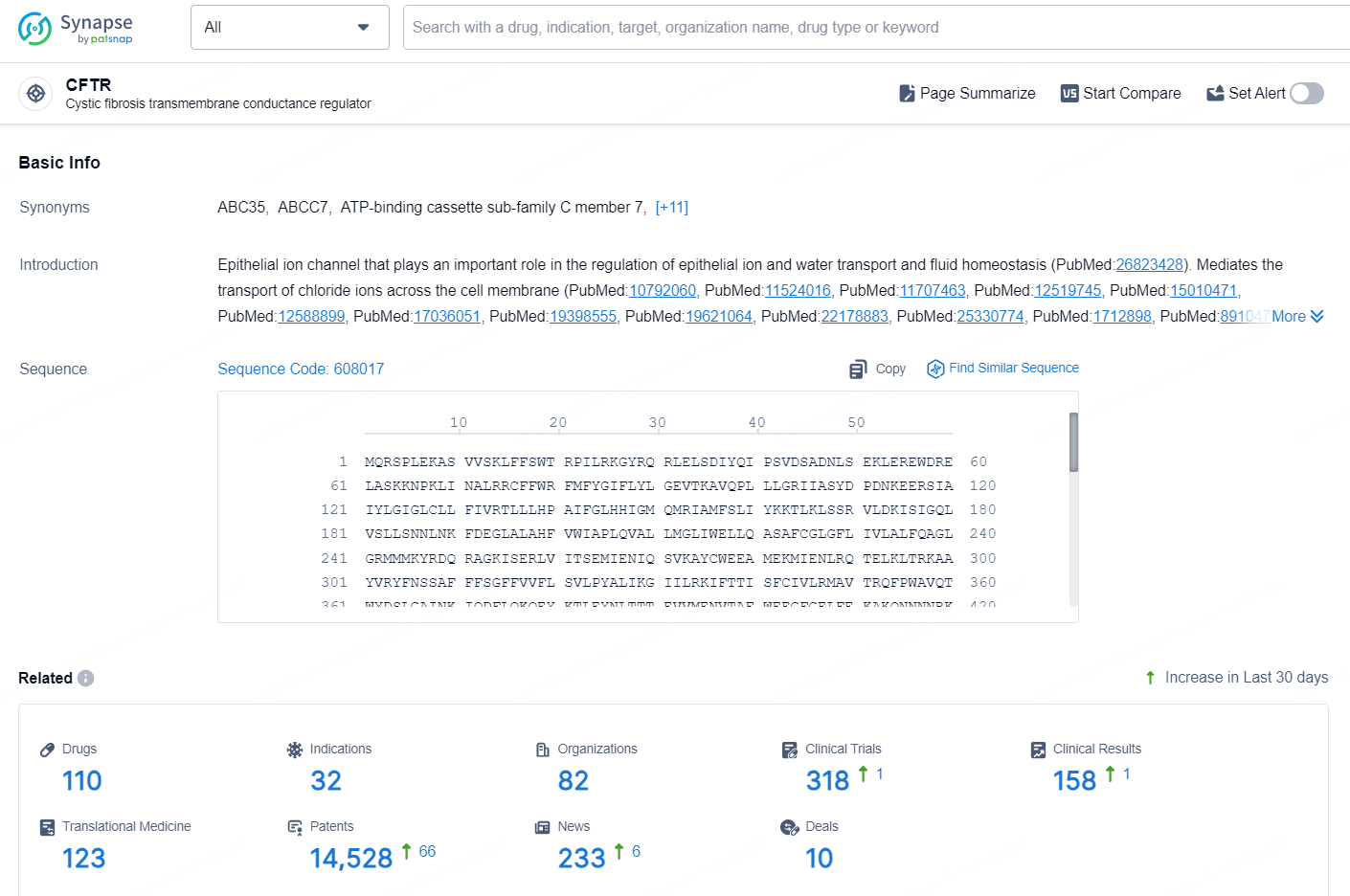
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 29, 2024, there are 110 investigational drugs for the CFTR targets, including 32 indications, 82 R&D institutions involved, with related clinical trials reaching 318, and as many as 14528 patents.
The drug RCT-2100 is a mRNA-based therapy that targets the CFTR gene. It is being developed for the treatment of cystic fibrosis, a genetic disorder that affects the respiratory and digestive systems. In addition to cystic fibrosis, RCT-2100 has the potential to treat other congenital disorders, digestive system disorders, and respiratory diseases. The drug is currently in the Phase 1 stage of development, which is the earliest phase in the clinical trial process.