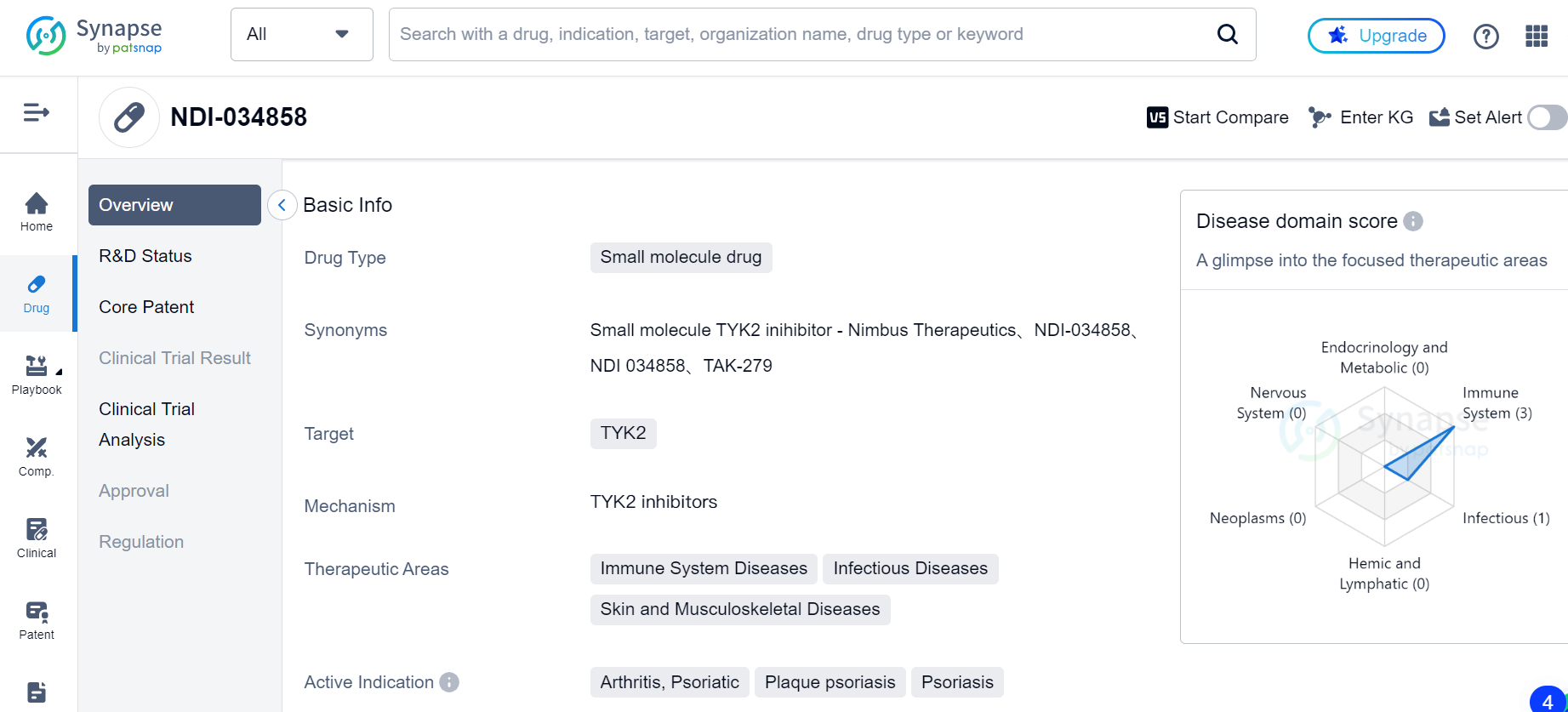
Discovery of a New Drug for Inflammatory and Autoimmune Diseases: TAK-279
Scientists have discovered a new drug candidate, TAK-279, which is a highly selective inhibitor of TYK2 enzymatic activity. TYK2 is a key mediator of IL12, IL23, and type I interferon signaling, and these cytokines have been implicated in the pathogenesis of multiple inflammatory and autoimmune diseases such as psoriasis, rheumatoid arthritis, lupus, and inflammatory bowel diseases.
Discovery of TAK-279
TYK2 mediates the downstream intracellular signaling of pro-inflammatory cytokines IL12, IL23, and type I interferon, which play a critical role in the function of Th1 and Th17 cells and consequently play a key role in a range of autoimmune and chronic inflammatory diseases. Genome-wide association studies have identified lossof-function (LoF) mutations of TYK2 in humans, which have been shown to be protective for a number of inflammatory diseases. These LoF variants reduce the function of TYK2 to mediate cytokine signaling, which in turn confers protection against a number of diseases.
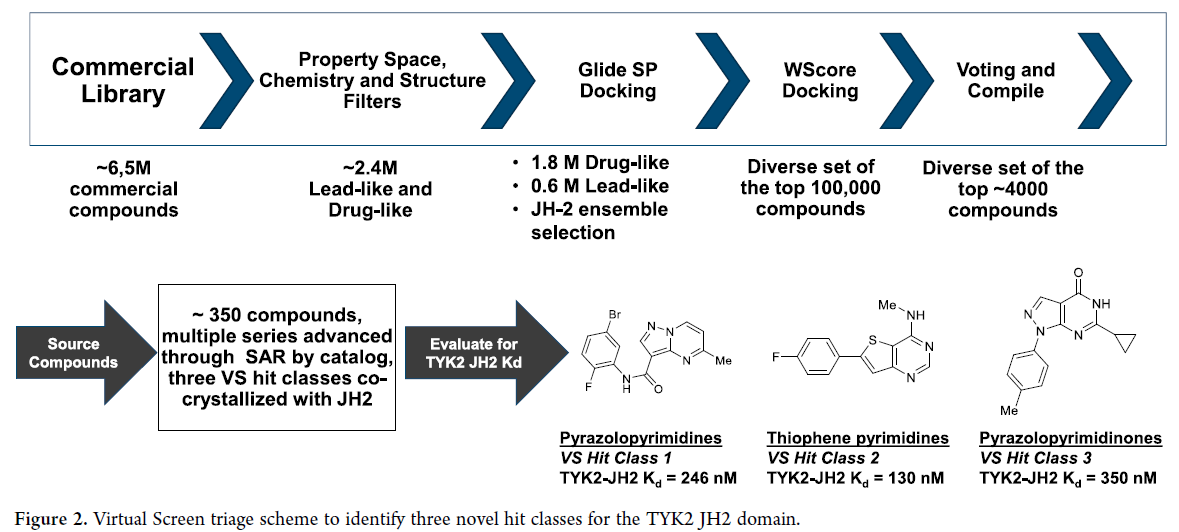
The discovery of TAK-279 was made possible by a computationally enabled design strategy, including the use of FEP+ (free energy perturbation). FEP+ is a computational method that calculates the free energy difference between two states of a molecule, such as a ligand bound to a protein and a ligand in solution. The authors used FEP+ to calculate the binding free energy of various pyrazolo-pyrimidine analogs to the TYK2 protein, and identified a pyrazolo-pyrimidine core that showed promising binding affinity. The pyrazolo-pyrimidine core was then optimized to develop TAK-279.
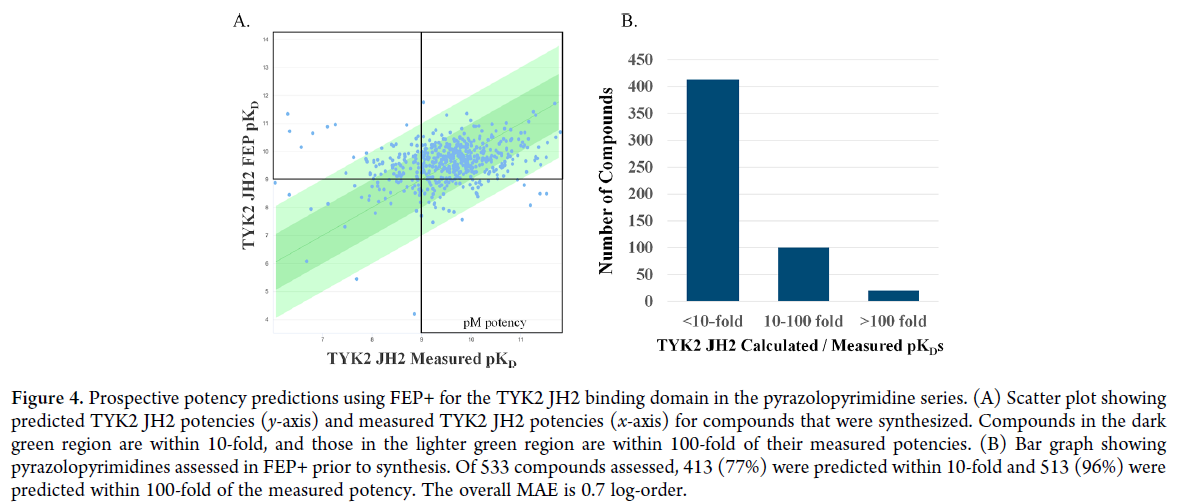
Optimization process
The optimization of the pyrazolo-pyrimidine core was achieved through a series of modifications to improve the potency and selectivity of the compound. The researchers used a combination of computational modeling and experimental assays to optimize the structure of the pyrazolo-pyrimidine core. They made modifications to the core structure, such as adding substituents and changing the position of the substituents, to improve the binding affinity and selectivity of the compound.
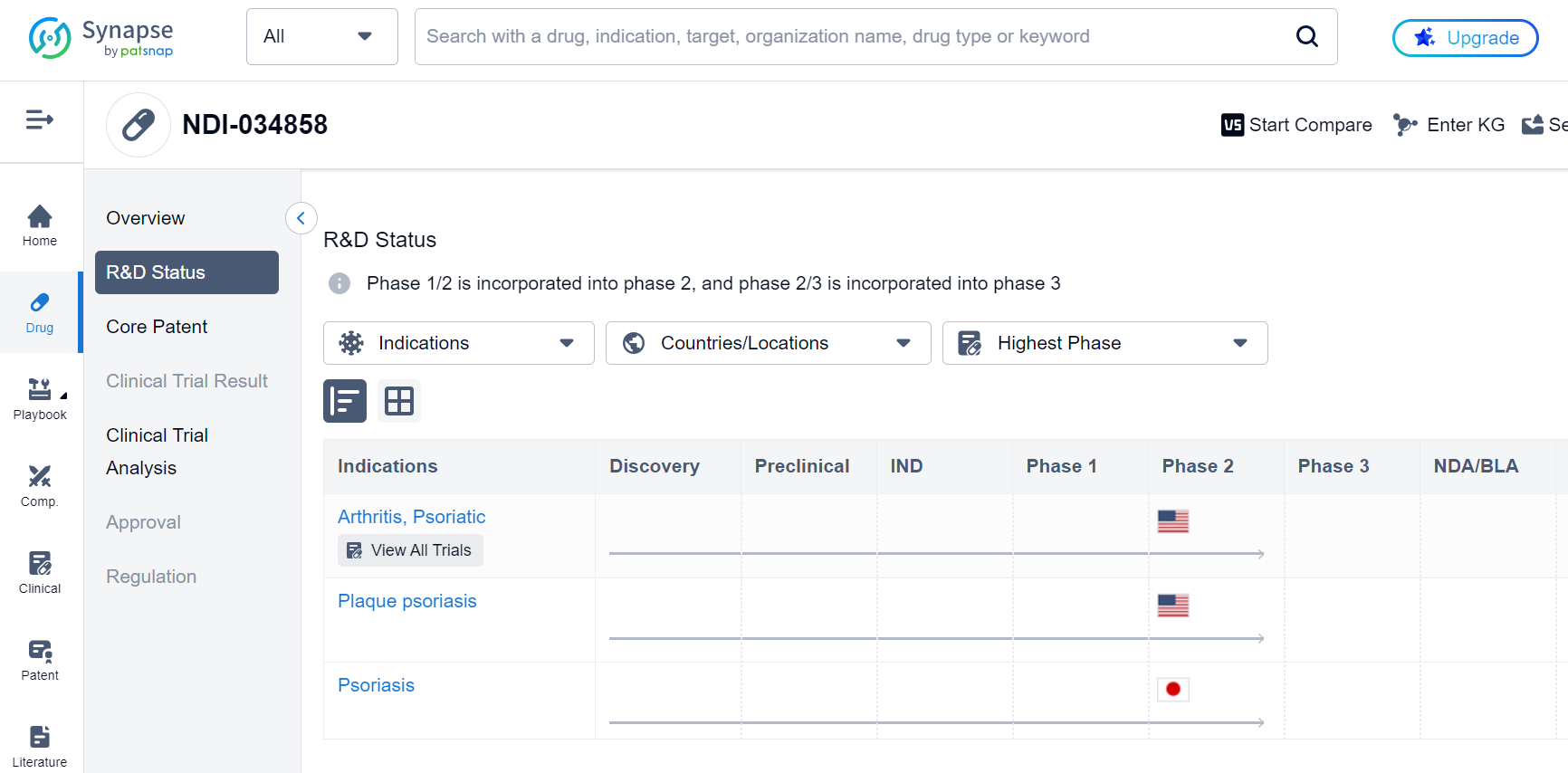
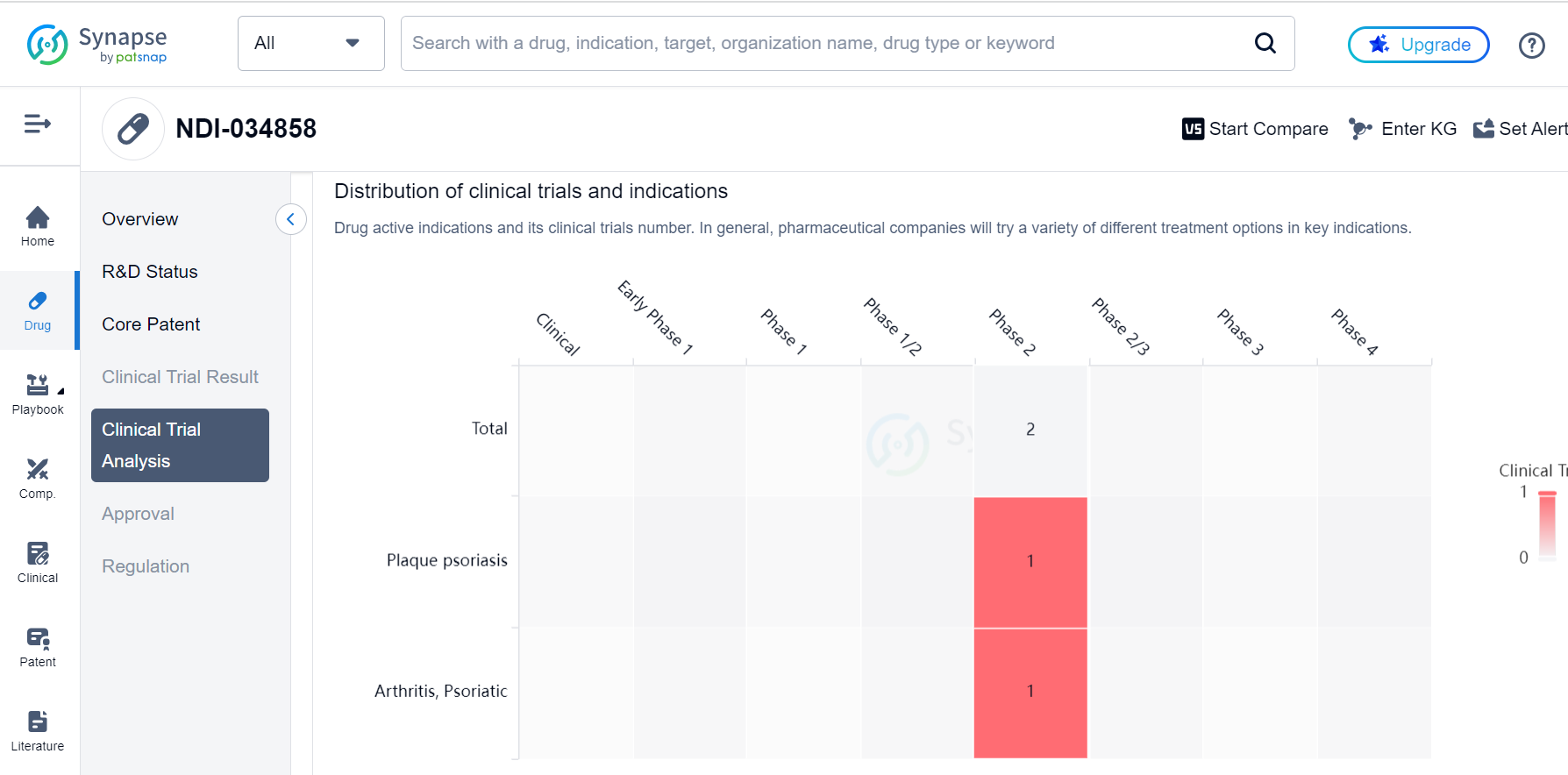
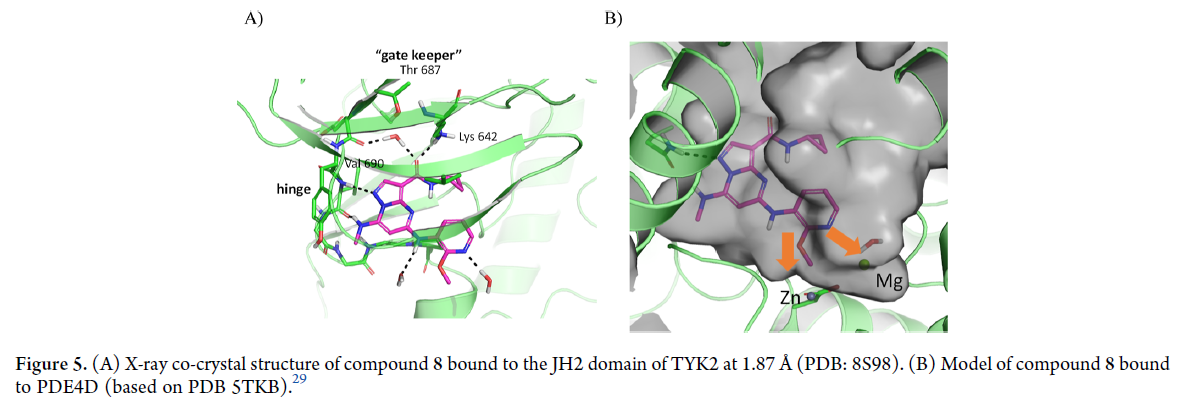
In addition, they also used X-ray crystallography to determine the binding mode of the compound to the TYK2 protein, which helped guide the optimization process. Through this iterative process of design and testing, the researchers were able to identify TAK-279, a potent and highly selective TYK2 inhibitor that is currently in Phase 2 clinical trials for the treatment of psoriasis and psoriatic arthritis, as shown in the Synapse database:
Selectivity and pharmacokinetics
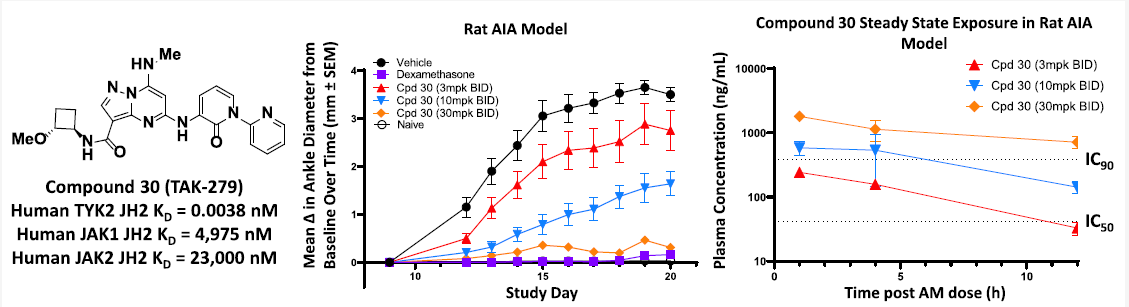
TAK-279 is highly selective for binding to the TYK2 JH2 domain (K D = 0.0038 nM) over the JAK1 and JAK2 JH2 domains (K D s = 4975 and 23,000 nM, respectively). It exhibits excellent selectivity over binding to the JAK1−3 JH1 domains and the TYK2 JH1 domain.
The results of PBMC pSTAT5 assays for TAK-279 and two other compounds are shown below. All three compounds were highly selective in the PBMC pSTAT5 assays, with TAK-279 showing the highest degree of selectivity. Specifically, TAK-279 had an IC50 value of 0.002 μM for TYK2 inhibition, while its IC50 values for JAK1, JAK2, and JAK3 inhibition were all greater than 10 μM. This indicates that TAK-279 is highly selective for inhibition over JAK1, JAK2, and JAK3 inhibition.
The in vivo efficacy of TAK-279 in various animal models of inflammatory and autoimmune diseases, including psoriasis and psoriatic arthritis has been tested in subsequent experiments. Specifically, in a mouse model of collagen-induced arthritis, TAK-279 demonstrated a dose-dependent inhibition of paw swelling and joint inflammation. Similarly, in a mouse model of experimental autoimmune encephalomyelitis (EAE), TAK-279 showed a dose-dependent inhibition of disease progression and reduced infiltration of immune cells into the central nervous system. Furthermore, in a mouse model of psoriasis, TAK-279 displayed a dose-dependent inhibition of skin inflammation and reduced levels of pro-inflammatory cytokines.
The nonclinical safety profile of TAK-279 was well characterized in a battery of in vitro and in vivo genetic toxicology, safety pharmacology, developmental/reproductive toxicology, and repeat-dose oral toxicology studies. The no observed adverse effect level (NOAEL) in the rat chronic toxicology study was 30 mg/kg/day, which was 30 times higher than the anticipated clinical dose. The NOAEL in the cynomolgus monkey chronic toxicology study was 10 mg/kg/day, which was 10 times higher than the anticipated clinical dose. Based on the rat and cynomolgus monkey chronic toxicology studies, adequate safety margins supported further clinical development of TAK-279.
The discovery of TAK-279 is a significant breakthrough in the treatment of inflammatory and autoimmune diseases. The drug candidate's excellent potency, off-target selectivity, ADME properties, and in vivo efficacy make it a promising therapeutic strategy for multiple autoimmune and immunological disorders.