Bringing KRAS G12C Inhibitors to Market: The Game Is On
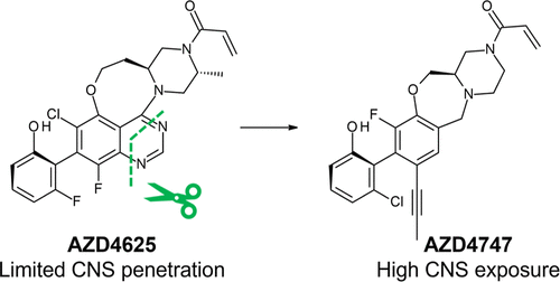
Recently, researchers from AstraZeneca have discovered a new drug candidate, AZD4747, that shows promise in treating tumors with the KRAS G12C mutation. This mutation limits the ability of the GTPase KRAS to hydrolyze GTP to the inactive GDP-bound state, resulting in the constitutive activation of downstream signaling pathways. The KRAS G12C mutation occurs with high frequency in lung adenocarcinomas and to a lesser degree in pancreatic and colorectal adenocarcinomas.
KRAS mutations are prevalent in many human cancers and are one of the most commonly mutated oncogenes. KRAS mutations have been detected in approximately 95% of pancreatic cancers, 40% of colorectal cancers, 25% of non-small cell lung cancers, and in thyroid, ovarian, bladder, lupus, breast, liver and other cancer types.
Global incidence rates of KRAS-positive cancers are rising significantly. Cases increased from 1.8 million in 2016 to over 2 million in 2020. Cases are projected to exceed 2.28 million by 2025 and 2.56 million by 2030. As of July 10, 2023, according to Synapse's Database, 4 new drugs targeting KRAS were added in the past 30 days, along with 6 new clinical trials and 55 new patents. For the latest updates on KRAS-targeting drugs in development, simply click the image below to register for free access to Synapse's latest intelligence.

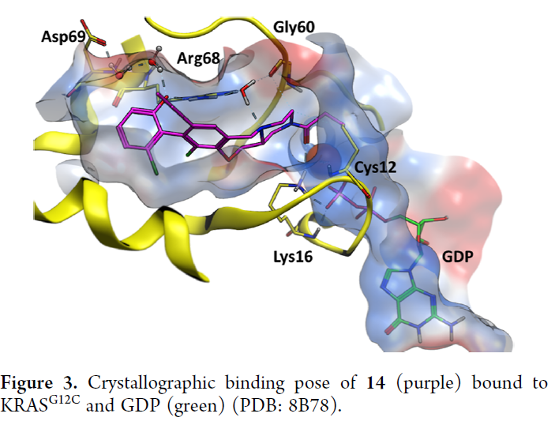
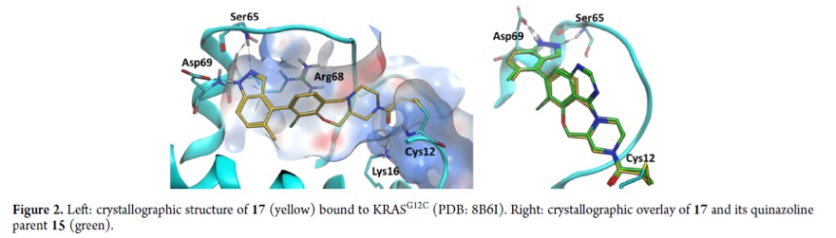
The AstraZeneca team use a structure-based drug design approach to identify AZD4747, which is a highly potent and selective inhibitor of KRAS G12C. The drug candidate was optimized for potency and drug metabolism and pharmacokinetics (DMPK) properties. The researchers used a crystal structure of AZD4747 bound to inactive KRAS G12C to determine its binding mode. The chlorophenol switch II group interacts with Arg68 and makes through-water interactions with Asp69. The C8 methylacetylene is solvent-exposed, while the oxazepane puckers in a manner to present a sp3-hybridized N piperazine atom to a bridging water. This neutral N atom stabilizes the water molecule, which itself is further stabilized into a tetrahedral geometry by interacting with Arg68 and Gly60.
AZD4747 forms a covalent bond with Cys12 and interacts with Lys16. It is highly selective for reaction with the cysteine in KRAS G12C over other cysteine-containing proteins and has a selective antiproliferative phenotype in cancer cells bearing the G12C mutation that led to strong tumor regressions in a mouse xenograft disease model.
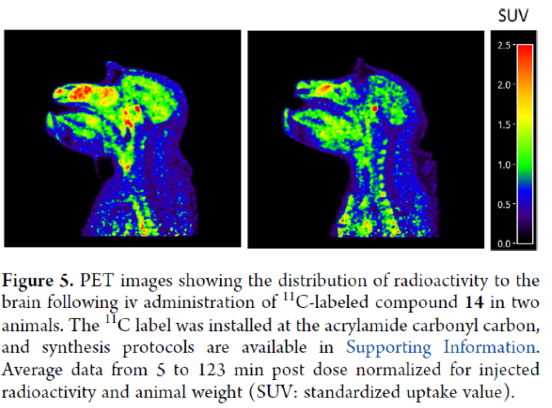
The researchers also found that AZD4747 displays ideal molecular properties to achieve drug exposure in the central nervous system (CNS), and its in vitro efflux potential for human P-gp and BCRP has been profoundly reduced. The mean in vivo Kpu,u obtained for dogs and monkeys are 0.7 and 1.6, respectively, indicating CNS availability relatively unimpaired by active efflux in these species. Based on these results, AZD4747 is proposed to be capable of crossing the intact blood-brain barrier in humans and thus demonstrates the potential to treat patients with CNS metastases.
This represents the latest effort by pharmaceutical companies to develop KRAS G12C inhibitors and bring them to the market. In fact, the race to develop KRAS G12C inhibitors is highly competitive, with multiple drugs in clinical trial. The first FDA-approved KRAS G12C inhibitor is sotorasib (Lumakras), which received accelerated approval in May 2021 for treating patients with locally advanced or metastatic NSCLC previously treated and with the KRAS G12C mutation. The approval was based on results from the CodeBreaK 100 trial, which demonstrated that sotorasib reduced tumors in 36% of patients, with a median duration of response of 10 months. Sotorasib is also being tested in clinical trials in combination with immunotherapy, chemotherapy, or targeted therapies for NSCLC and other KRAS G12C cancers.
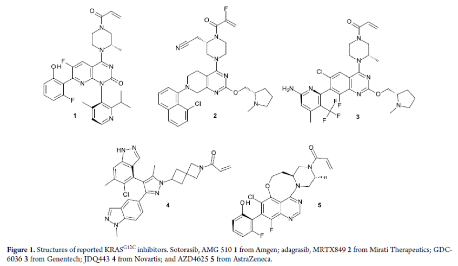
Currently, drug candidates like AMG510 and MRTX849 have already been approved, and many other inhibitors are in clinical stages worldwide. In addition to the published structures of Genentech's GDC-6036, Novartis' JDQ443, and AstraZeneca's AZD4625, there are also ongoing clinical trials for LY3537982 (Eli Lilly), BI 1823911 (Boehringer Ingelheim), and D-1553 (InventisBio) whose structures have not yet been disclosed. Early-stage molecules LY3499446 (Eli Lilly) and JNJ-74699157 (Johnson & Johnson) were discontinued due to toxicity. Other KRAS subtypes like G12D, G12V and G13D are also being actively pursued, as are panKRAS and RAS-on inhibitors.
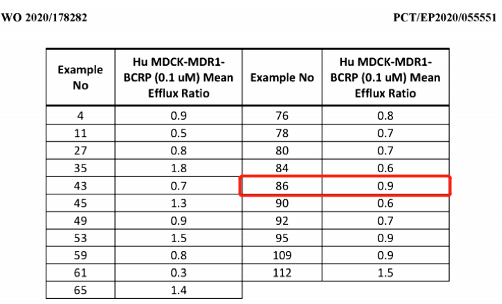
Four WO patents turned up from searching the Synapse patent database using keywords KRAS and AstraZeneca: WO2020178282, WO2019215203, WO2019110751, and WO2018206539. The last three patents are unlikely related to brain-penetrant KRAS inhibitors. Embodiments 85 and 86 within WO2020178282 refer to the compound AZD4747, a racemic mixture of two enantiomers. Only the activity and efflux data of compound 86 were measured, suggesting it represents AZD4747. MDCK_MDR1_BCRP cells express two efflux pumps that transport compounds across the blood-brain barrier. Compounds with efflux ratios ≤2 in this assay have potential as brain-penetrant agents.
Compounds with this potential are shown below, including compound 86, determined to have balanced drug properties based on other evaluations. WO2020178282's earliest priority date is March 5, 2019. It was filed March 3, 2020 and published September 10, 2020. The expected expiration date for CN113508118A, the Chinese publication, is March 3, 2040.
The KRAS G12C inhibitor sotorasib has shown promising anti-tumor activity in clinical trials for KRAS-mutant non-small cell lung cancer (NSCLC). However, these trials have excluded patients with central nervous system (CNS) metastases, limiting data on sotorasib's ability to penetrate the brain. A retrospective analysis by Sabari et al. and a review of preclinical data suggest the experimental drug adagrasib, which also targets KRAS G12C, may have CNS activity.
In preclinical models of meningitis, adagrasib penetrated into cerebrospinal fluid and led to tumor regression and longer survival. In two NSCLC patients with untreated meningitis, adagrasib achieved cerebrospinal fluid concentrations exceeding the IC50 values needed to inhibit tumor growth. Both patients had corresponding brain metastases, suggesting adagrasib may have clinical activity against brain metastases. However, some critics argue the limited data from only two patients is insufficient, as treating and preventing brain metastases is critical for patient outcomes.
These findings support further study of adagrasib in KRAS G12C NSCLC patients with untreated brain metastases. A phase I/II trial (NCT03785249) is recruiting 822 late-stage solid tumor patients with the KRAS G12C mutation to evaluate adagrasib's safety, tolerability, drug levels, molecular effects, and clinical activity, with results expected in September 2024.
In early clinical trials of another experimental KRAS G12C inhibitor, D-1553, one of three patients with baseline brain metastases achieved a partial response, for a 17% objective response rate. In this patient, metastatic lesions larger than 20mm shrank by nearly half. The other two patients had stable disease, for a 100% disease control rate.
Some patients with cancer brain metastases may have a compromised blood-brain barrier, allowing some compounds to penetrate the brain even if they did not in preclinical models. AstraZeneca, which is developing adagrasib, has successfully developed other brain-penetrating drugs that could benefit these patients. Preclinical and limited clinical data suggest the KRAS G12C inhibitors adagrasib and D-1553 may have activity against brain metastases in NSCLC. Ongoing and future clinical trials will provide more robust evidence on their efficacy in this critical patient population.