Domain Therapeutics and Chime Biologics Ink Deal to Advance CCR8 Antibody for Cancer Immunotherapy
Domain Therapeutics and Chime Biologics have publicized the execution of a contract for manufacturing services. The agreement specifies the fabrication of Domain Therapeutics' leading anti-CCR8 monoclonal antibody, known as DT-7012, which targets the depletion of Tregs. The goal of this production stage is to offer a potent treatment option to the global cancer patient community.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Pursuant to the contractual obligations, Chime Biologics is committed to facilitating consistent cellular line creation and the production of the DT-7012 candidate, bolstering the upcoming clinical assessments in key nations. Chime Biologics boasts its premier worldwide plant, equipped with disposable bioprocessing apparatus that aligns with the universal cGMP requisites and boasts a robust history of satisfactory audits.
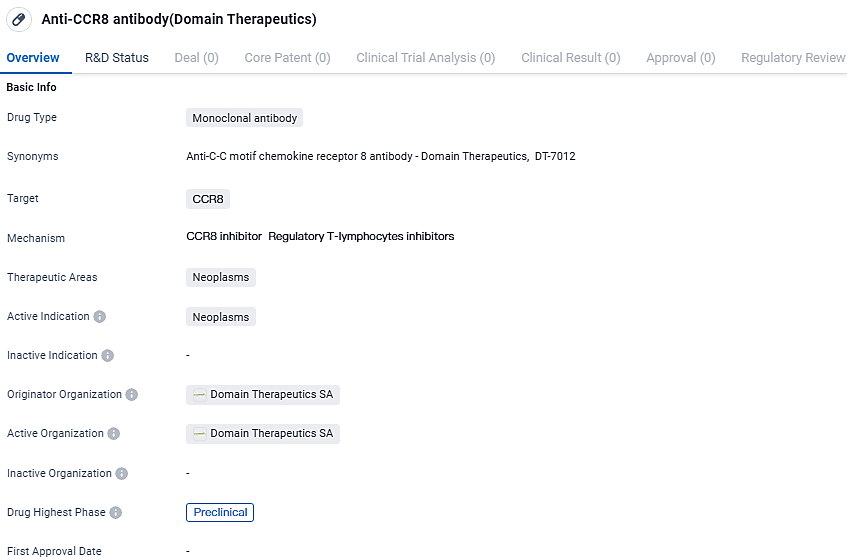
DT-7012 is an innovative monoclonal antibody targeted against CCR8 that significantly depletes Tregs present within tumors, these cells being prominent in suppressing immune functions. The solo application of the anti-CCR8 monoclonal antibody has revealed a distinctive capability to counteract tumors.
When juxtaposed with peer CCR8-targeting antibodies currently undergoing clinical trials, DT-7012 has manifested unparalleled potential, forging a path for successful GPCR-oriented immunotherapy approaches designed to invigorate the immune response against tumors in patients who have not benefited from alternative treatments. It is anticipated that Phase I clinical evaluations of DT-7012 will commence at the onset of 2025 for solid malignancies and midway through 2025 for cutaneous T-cell lymphoma.
Dr. Jimmy Wei, holding the presidential position at Chime Biologics, stated, “Anticipating the advancement of this pivotal alliance is exciting, blending the expertise of Domain in pioneering its anti-CCR8 antibody candidate with Chime Biologics' proficiency from CLD to full-scale production, a significant move in progressing DT-7012's application across a spectrum of cancer types.”
Expressing views on the collaboration, Stephan Schann, the Chief Scientific Officer at Domain Therapeutics, communicated: “The groundbreaking GPCR-focused immunotherapeutic we're developing holds extensive promise in unleashing the capabilities of the immune system to combat oncological conditions, potentially benefiting a global patient demographic. At Domain, we hold an unwavering commitment to precision in research and trailblazing innovation, and we actively seek cooperative endeavors with entities that echo our earnest commitment to propel the frontiers of immuno-oncology.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
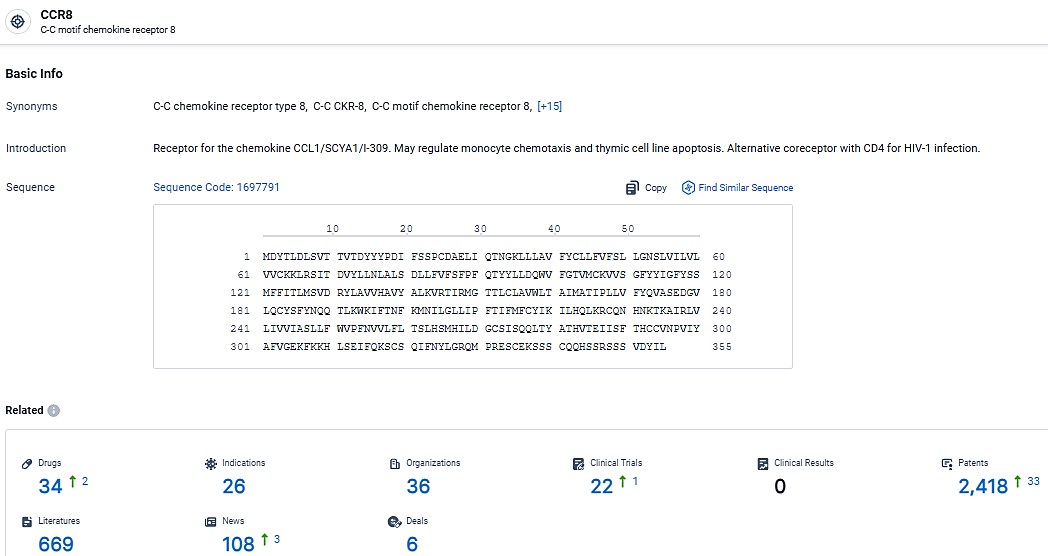
According to the data provided by the Synapse Database, As of March 13, 2024, there are 34 investigational drugs for the CCR8 target, including 26 indications, 36 R&D institutions involved, with related clinical trials reaching 22, and as many as 2418 patents.
DT-7012 is a monoclonal antibody that targets CCR8 and aims to treat neoplasms. It is currently in the preclinical phase, and further research is needed to assess its potential as a therapeutic option for neoplasms.