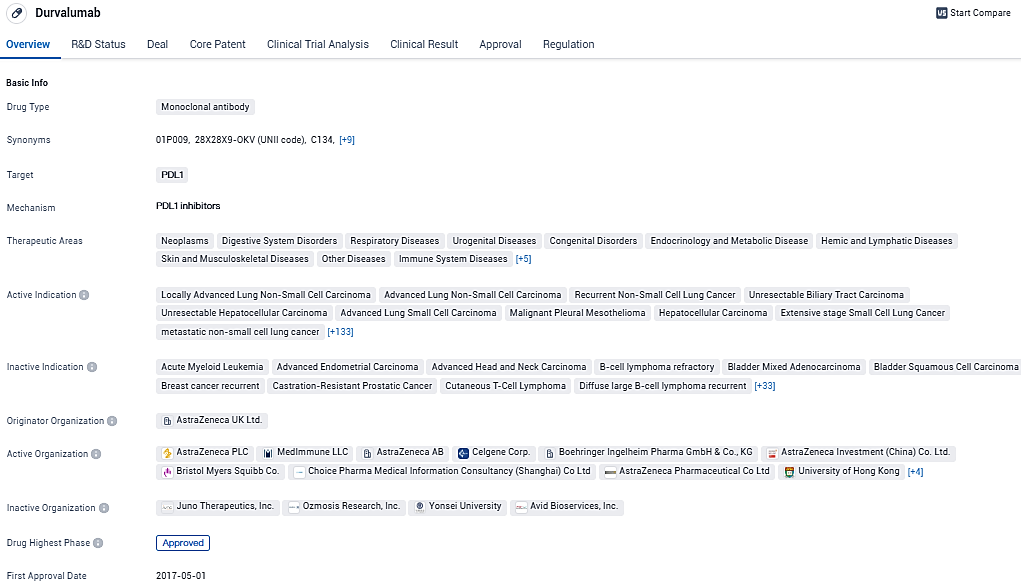
Durvalumab (IMFINZI®) plus TACE and bevacizumab reduced liver cancer progression or mortality risk by 23%
The EMERALD-1 Phase III trial provided encouraging outcomes, showing that AstraZeneca's IMFINZI (durvalumab), used alongside TACE and bevacizumab, statistically and notably increased the primary endpoint of progression-free survival in patients who have hepatocellular carcinoma and are eligible for embolization, compared to the use of TACE by itself.
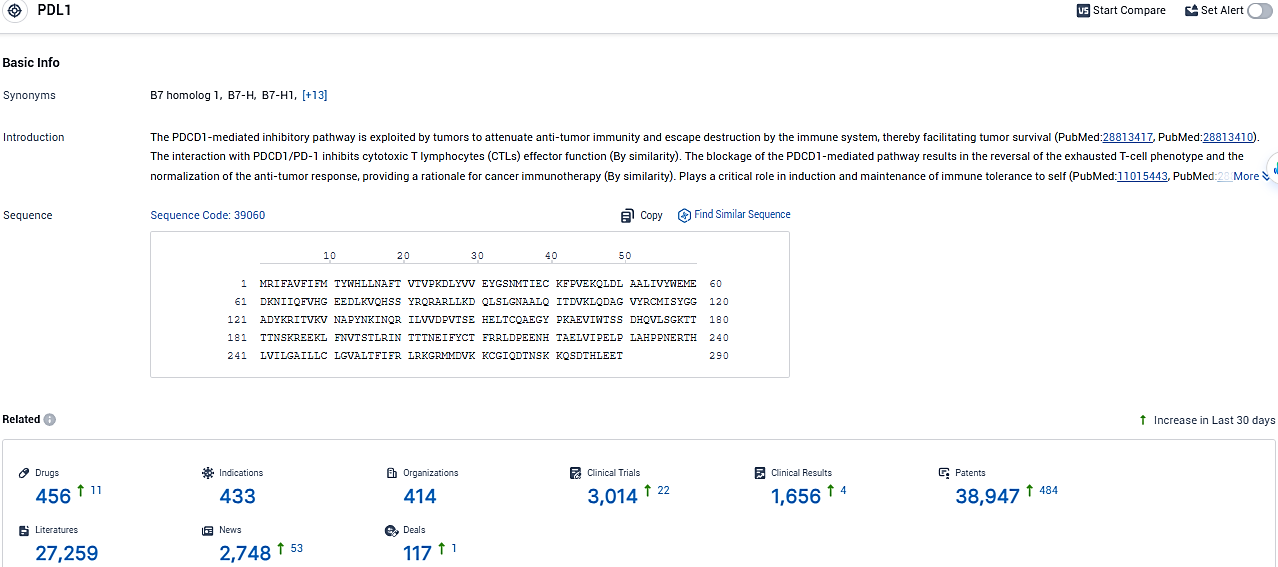
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Roughly 20-30% of patients diagnosed with HCC, the predominant form of liver cancer, qualify for embolization treatment. This technique cuts off the tumor's blood supply and can directly dispense chemotherapy or radiation therapy to the liver. Notwithstanding its status as the usual medical routine, the majority of patients subjected to embolization face disease relapse or advancement in approximately eight months.
The EMERALD-1 trial revealed a 23% reduction in the risk of disease deterioration or fatality when treated with IMFINZI in combination with TACE and bevacizumab, compared to TACE alone. Patients subjected to the combined IMFINZI therapy reported a median PFS of 15 months compared to 8.2 months with TACE. The observed PFS benefit was uniformly prevalent across significant preestablished subdivisions. The supplementary endpoint of time to progression bolsters the clinical advantage of IMFINZI plus TACE and bevacizumab, with a median TTP of 22 months over 10 months for TACE.
Bruno Sangro, MD, PhD, Chief of the Liver Unit and Medical Professor at Clínica Universidad de Navarra, conveyed: “Integrating durvalumab with TACE and bevacizumab lowered the threat of disease advancement or death by twenty-three per cent for liver cancer patients qualifying for embolization. This confirms the first such instance wherein a systemic treatment coupled with TACE can significantly enhance this clinically pertinent outcome in earlier stages of the disease.”
Susan Galbraith, the Executive VP of Oncology R&D at AstraZeneca, expressed: “Thanks to the IMFINZI-centered therapy, liver cancer patients qualifying for embolization experienced nearly seven additional months before their disease worsened. We're engaged in consultations with international regulatory agencies regarding these promising EMERALD-1 findings, while eagerly awaiting the trial's ultimate overall survival outcomes.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 25, 2024, there are 456 investigational drugs for the PD-L1 target, including 433 indications, 414 R&D institutions involved, with related clinical trials reaching 3014, and as many as 38947 patents.
IMFINZI (durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumor's immune-evading tactics and releasing the inhibition of immune responses. IMFINZI is approved in combination with chemotherapy (gemcitabine plus cisplatin) in locally advanced or metastatic biliary tract cancer and in combination with IMJUDO (tremelimumab-actl) in unresectable HCC in the US, EU, Japan, China.