Easily Obtain Gene Sequences by Using Gene Names in The Patsnap Bio Database
Acquiring the sequence of a gene founded on its title is feasible and this article introduces one uncomplicated and swift technique that is consistently utilized. The Patsnap Bio Database endorses an array of retrieval methodologies encompassing general sequences, motif sequences, antibody sequences (CDRs), fragment sequences, combination sequences, and drug-gene sequences. Crucially, it furnishes provisions for gene name inquiries. Kindly follow this link for gratuitous access to the Patsnap Bio Sequence Database.
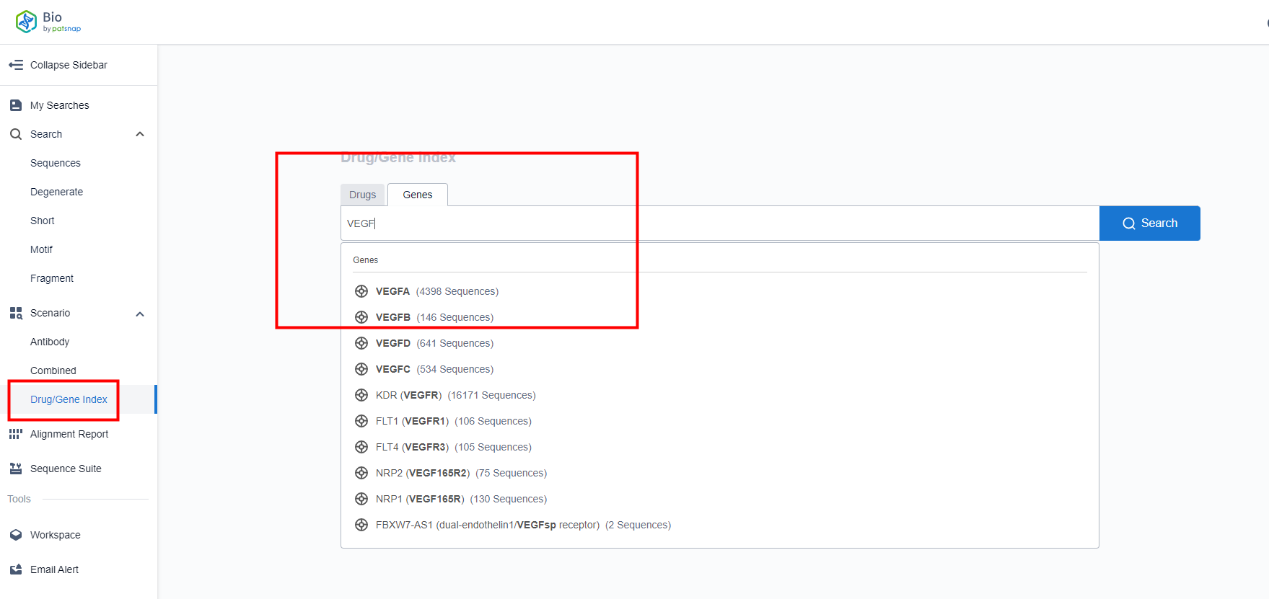
Described below are the steps for the search process: Primarily, accede to the Patsnap Bio Database and click on "Drug/Gene Search" on the left sidebar. Subsequently, input the gene name within the search box where a dropdown menu will suggest various gene names. From these suggestions, pinpoint and choose the desired gene. Click on the "Search" button to commence the search.
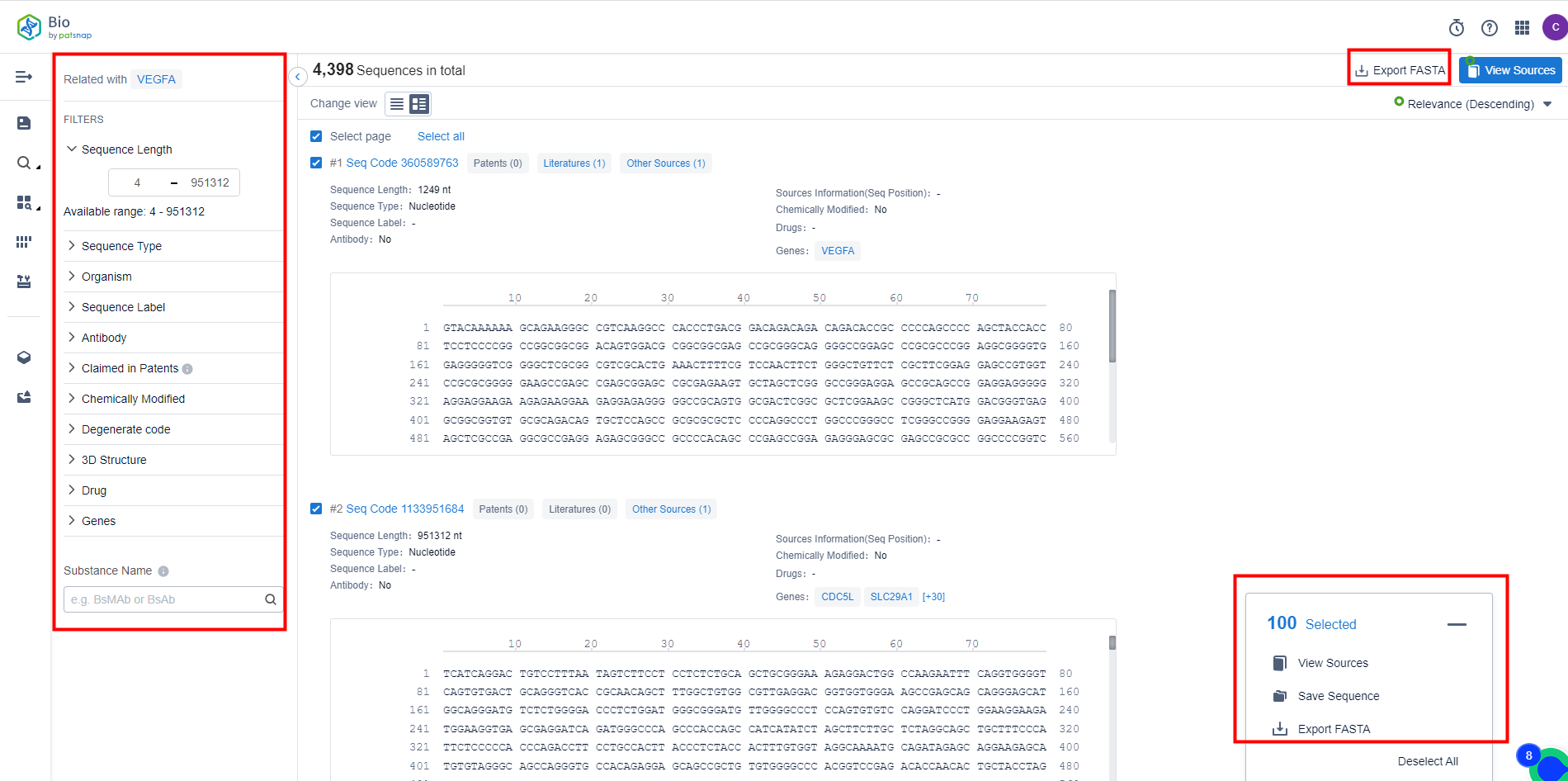
Secondarily, following the receipt of the search outcomes from the Patsnap Bio Database, it is possible to employ further filters to fine-tune the results. These filters may comprise sequence length, sequence type (DNA or protein), and specific species; eventually enabling the identification of the most pertinent sequences. Data outcomes support the exportation of FASTA files and the saving of sequences. Further, there are alternatives available to investigate public sources for additional data.
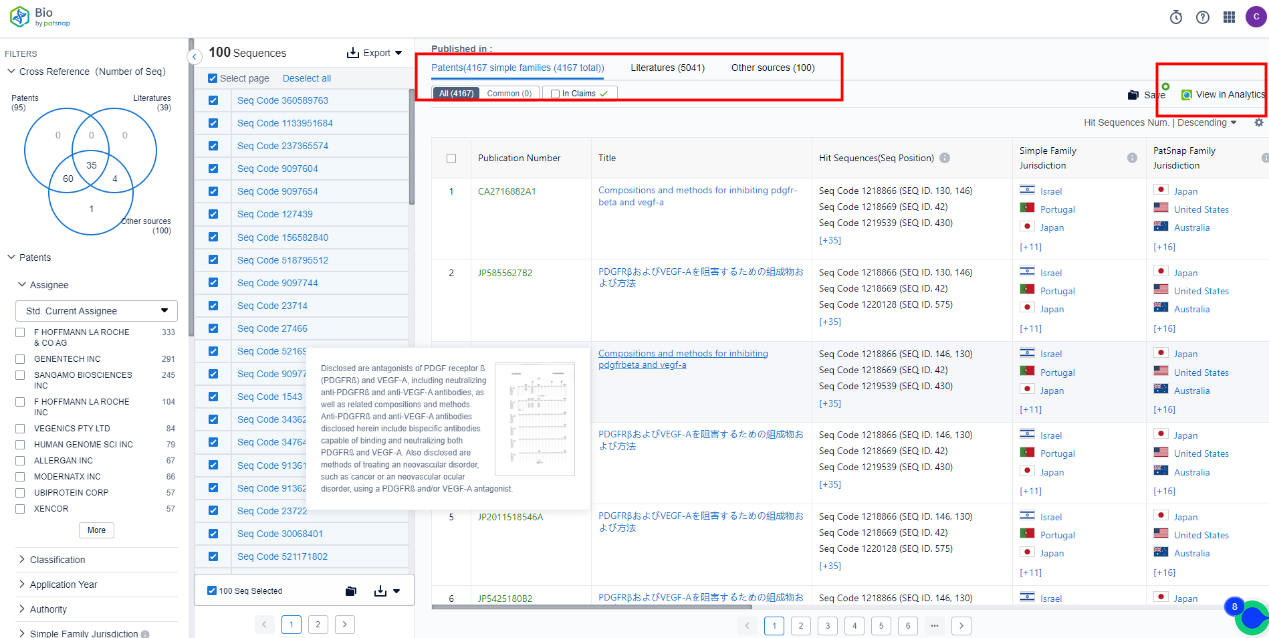
Thirdly, the search outcomes are also linked to the patent database providing direct access to patent specifics.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is currently available to utilize the Bio biological sequence database: https://bio-patsnap-com.libproxy1.nus.edu.sg. Act now to expedite your gene sequence search tasks.