Eirion Therapeutics Begins First Human Trials for Topical ET-02 in Androgenic Alopecia Treatment
Eirion Therapeutics Inc. has reported the enrollment of the initial participant in a Phase 1 clinical study aimed at assessing the safety of their proprietary topical medication, ET-02, for the treatment of androgenic alopecia. This placebo-controlled, double-blind trial seeks to evaluate the safety profile of ET-02 when applied once daily over a 28-day period. The trial plans to enlist around 24 participants across three research sites within the United States.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
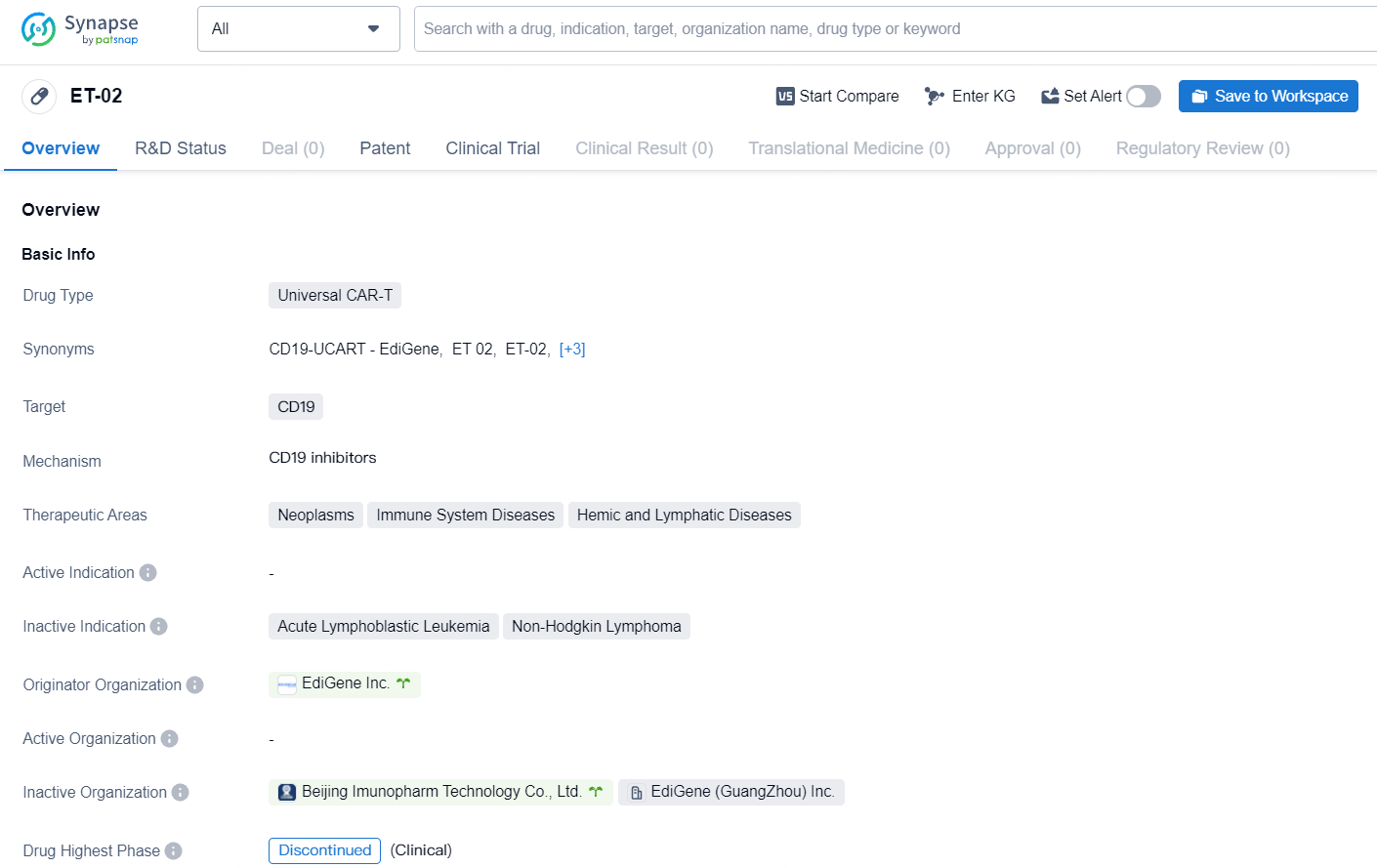
ET-02 operates with a unique mechanism of action aimed at reactivating hair follicle stem cells that have become defective or inactive in men experiencing age-related hair loss, restoring them to their normal function. These hair follicle stem cells are crucial as they act as “master control switches” for hair growth. By reactivating these stem cells, it is anticipated that normal hair growth can be resumed. ET-02 addresses what the Company identifies as the root cause of androgenic alopecia, with expectations that this treatment could be notably more effective than current treatments that do not directly impact hair follicle stem cells.
"Considering its hypothesized mechanism, ET-02 could represent a significant advancement over existing pharmaceutical options for treating androgenic alopecia," stated Jerry Shapiro, MD, a renowned expert in hair loss treatment and Professor of Dermatology at New York University Grossman School of Medicine.
Jon Edelson, MD, CEO and President of Eirion, remarked, "A controlled pre-clinical study of ET-02 on 90 human scalp tissue grafts from men with intermediate-stage androgenic alopecia exhibits this pharmaceutical’s potential in restoring hair growth and normalizing hair follicle structure and function. Due to its distinct mechanism, we believe ET-02 not only has the potential to treat but also to prevent androgenic alopecia."
In contrast to some other market-available treatments, ET-02 does not target hormonal pathways and is not expected to cause the side effects commonly associated with those treatments, such as sexual dysfunction.
"ET-02 complements our other two aesthetic bio-pharmaceutical products under development, both of which are potential treatments for facial wrinkles. Together, these three product candidates establish a strong and promising pipeline for innovation in aesthetic medicine," added Dr. Edelson.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
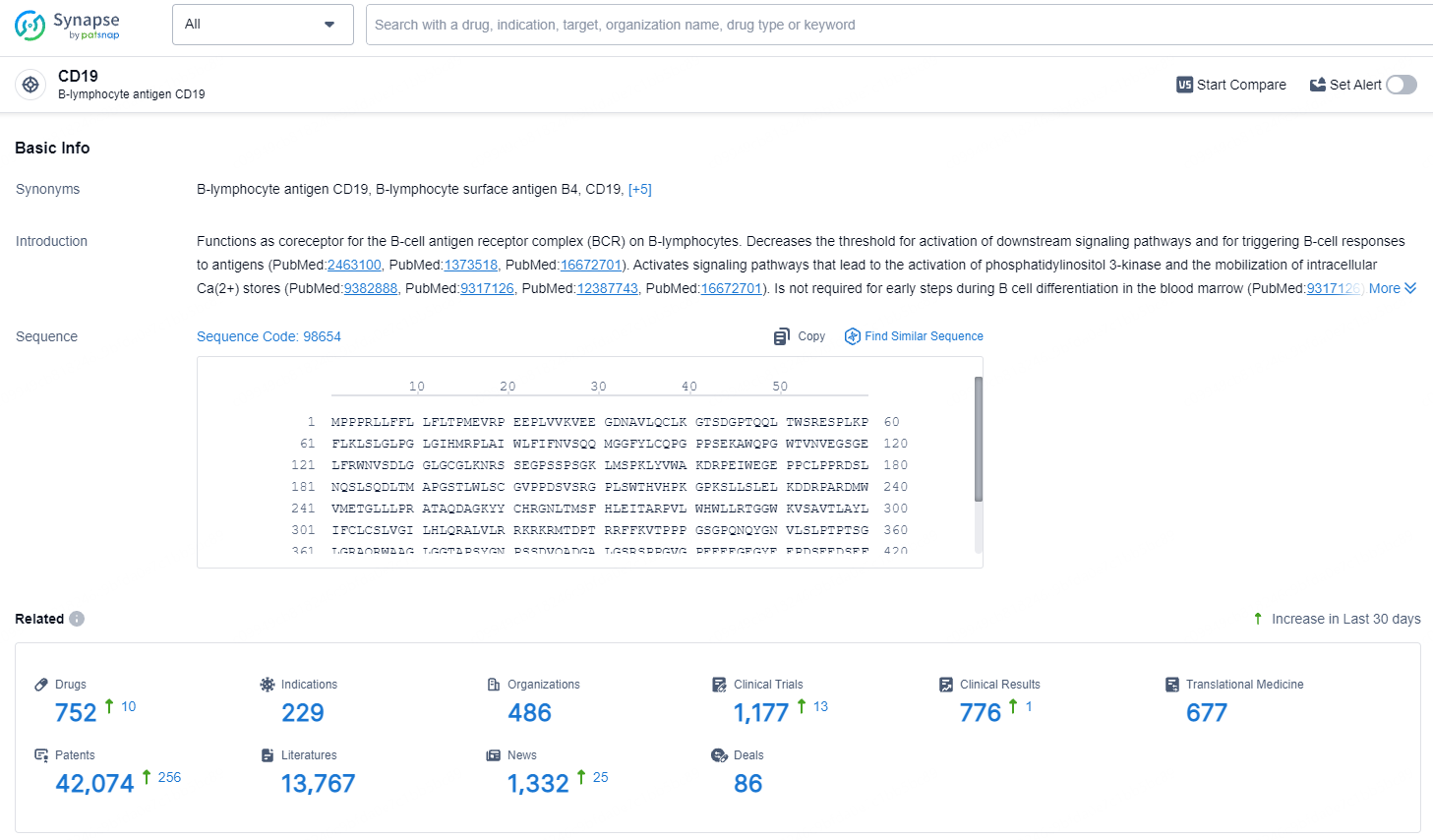
According to the data provided by the Synapse Database, As of July 8, 2024, there are 752 investigational drugs for the CD19 target, including 229 indications, 486 R&D institutions involved, with related clinical trials reaching 1177, and as many as 42074 patents.
ET-02 targets of CD19 for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. However, the development of the drug has been discontinued in both the global and Chinese markets.