What are the prospects for the AOC therapy that boosted Avidity by 33%?
On June 12, 2024, Avidity Biosciences, a U.S. publicly traded company, announced the latest data from the Phase 1/2 clinical trial FORTITUDE that investigates AOC1020 for treating FSHD. The stock price soared by 33% on the same day, reaching a market valuation of $3.7 billion; AOC1020 is the company's second product in development, following the initiation of a crucial Phase 3 clinical trial for their first pipeline product, AOC 1001. AOC 1020 targets DUX4, confirming that it can significantly downregulate DUX4-related genes.
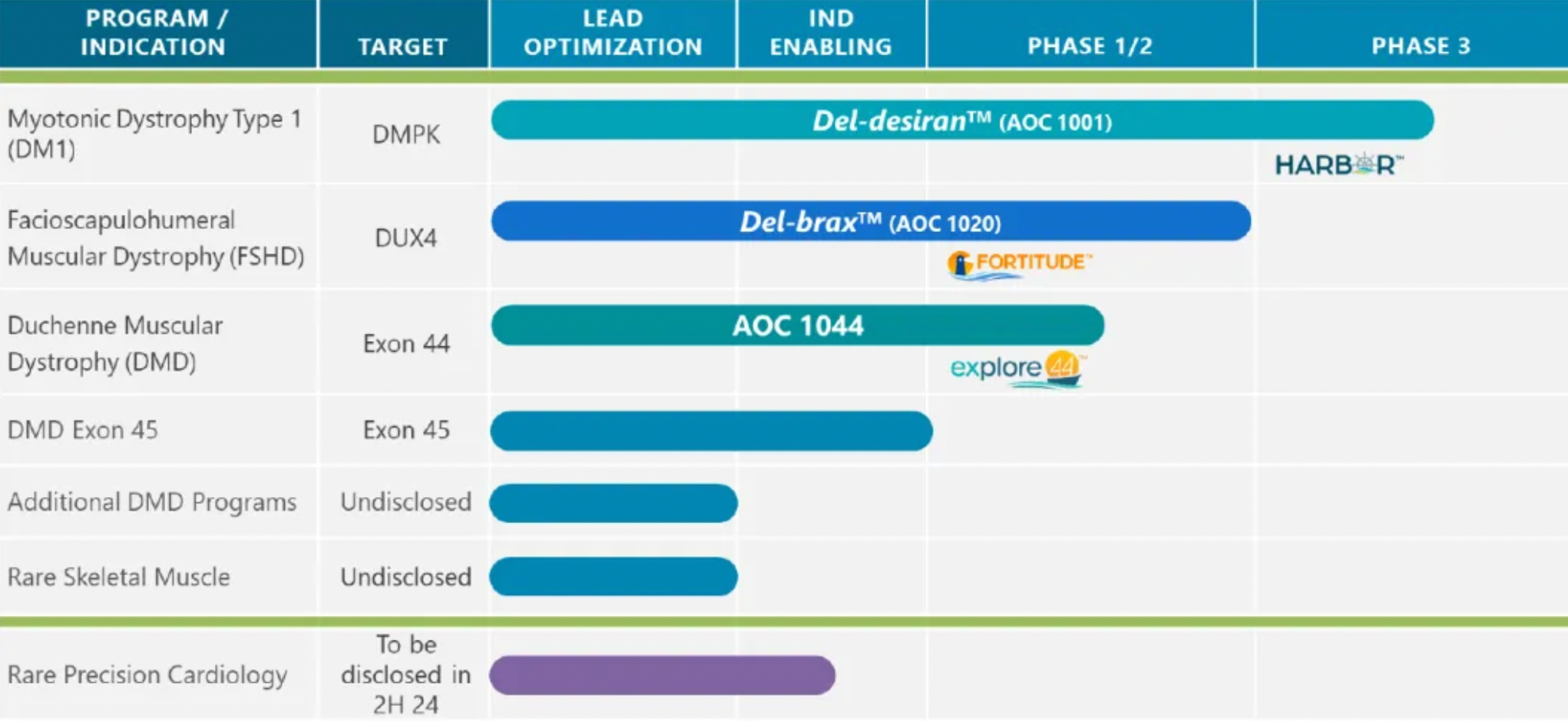
In the Phase 1/2 FORTITUDE trial, the most recent data revealed that treatment with AOC 1020 resulted in a significant reduction in DUX4-regulated genes, which is considered the underlying cause of Facioscapulohumeral Muscular Dystrophy (FSHD). Coupled with strong safety and tolerability profiles, these findings further support the progression of this therapeutic approach. FORTITUDE is a randomized, double-blind, placebo-controlled trial that assessed the administration of single and multiple doses of AOC 1020 or del-brax in approximately 39 adult FSHD patients. The trial examined the clinical activity of the treatment, including measurements of functionality and muscle strength, as well as patient-reported outcomes and quality of life assessments.
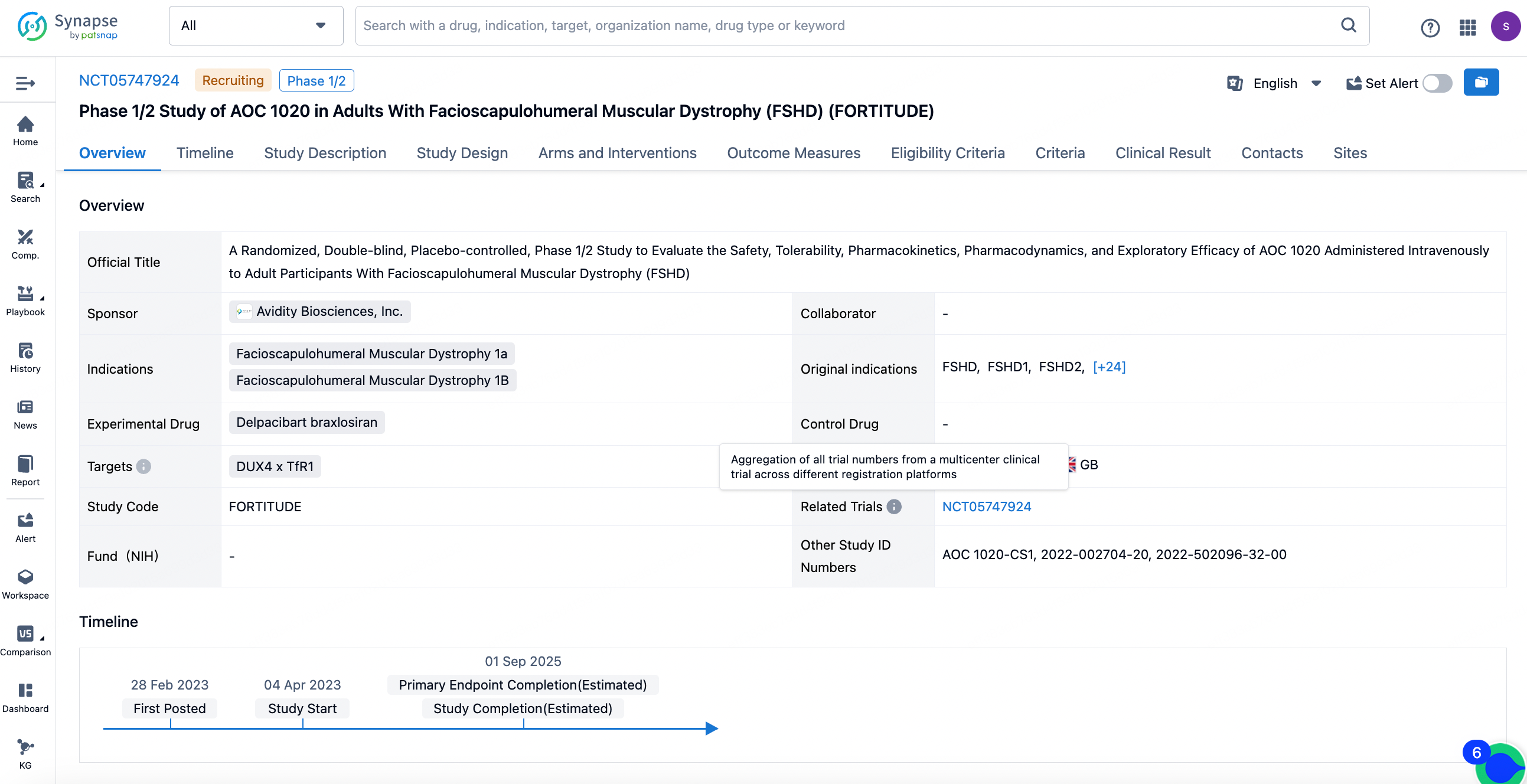
At the 31st annual FSHD Society International Research Conference held in Denver, Colorado on June 13-14, preliminary data included the safety and tolerability for patients in both the 2 mg/kg and 4 mg/kg groups after four months of treatment. The data for 12 participants in the 2 mg/kg group were assessed, encompassing DUX4 regulatory genes, circulating biomarkers, and muscle strength and function. As shown in the figure, in this smaller subgroup, there was an observation of an average reduction of over 50% in the expression of DUX4 regulatory genes across multiple panels in muscle tissue.
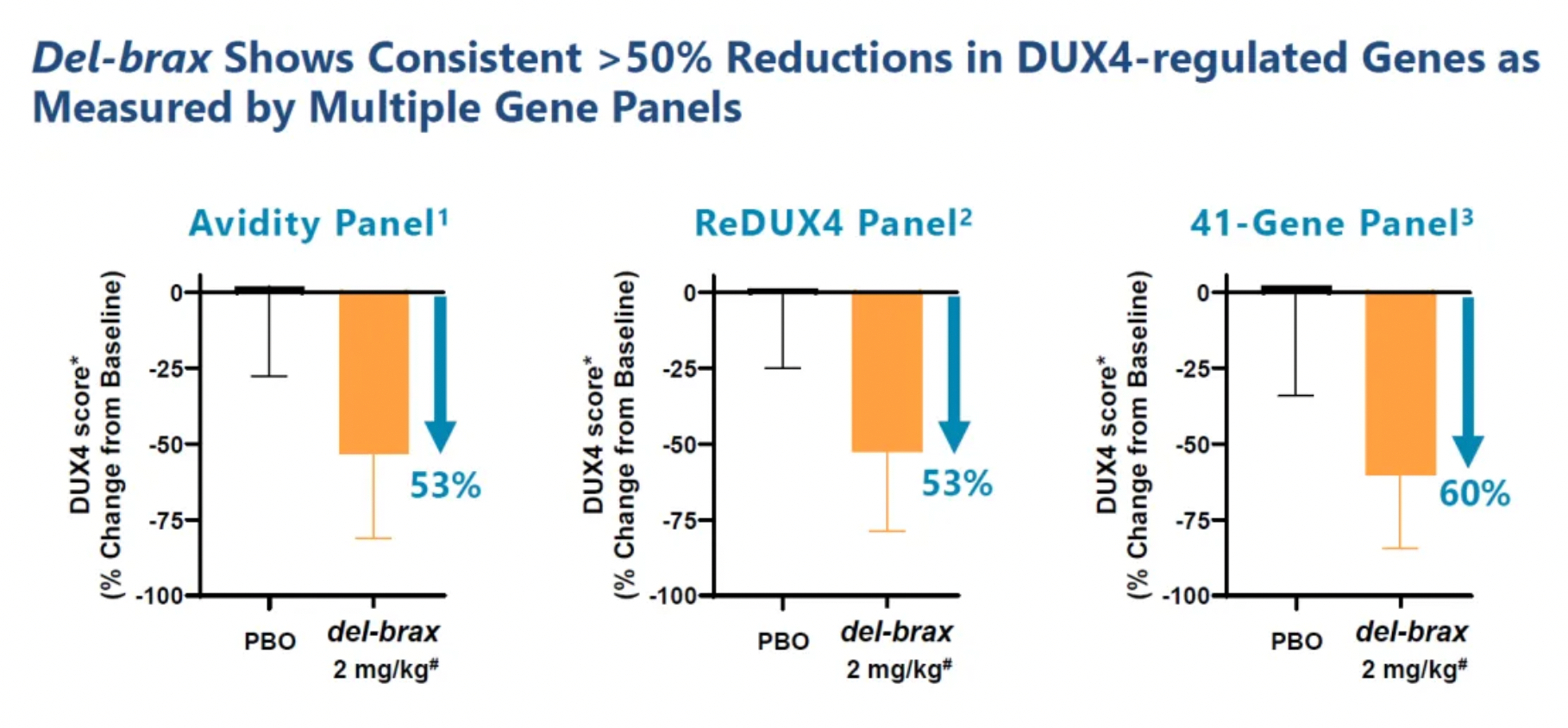
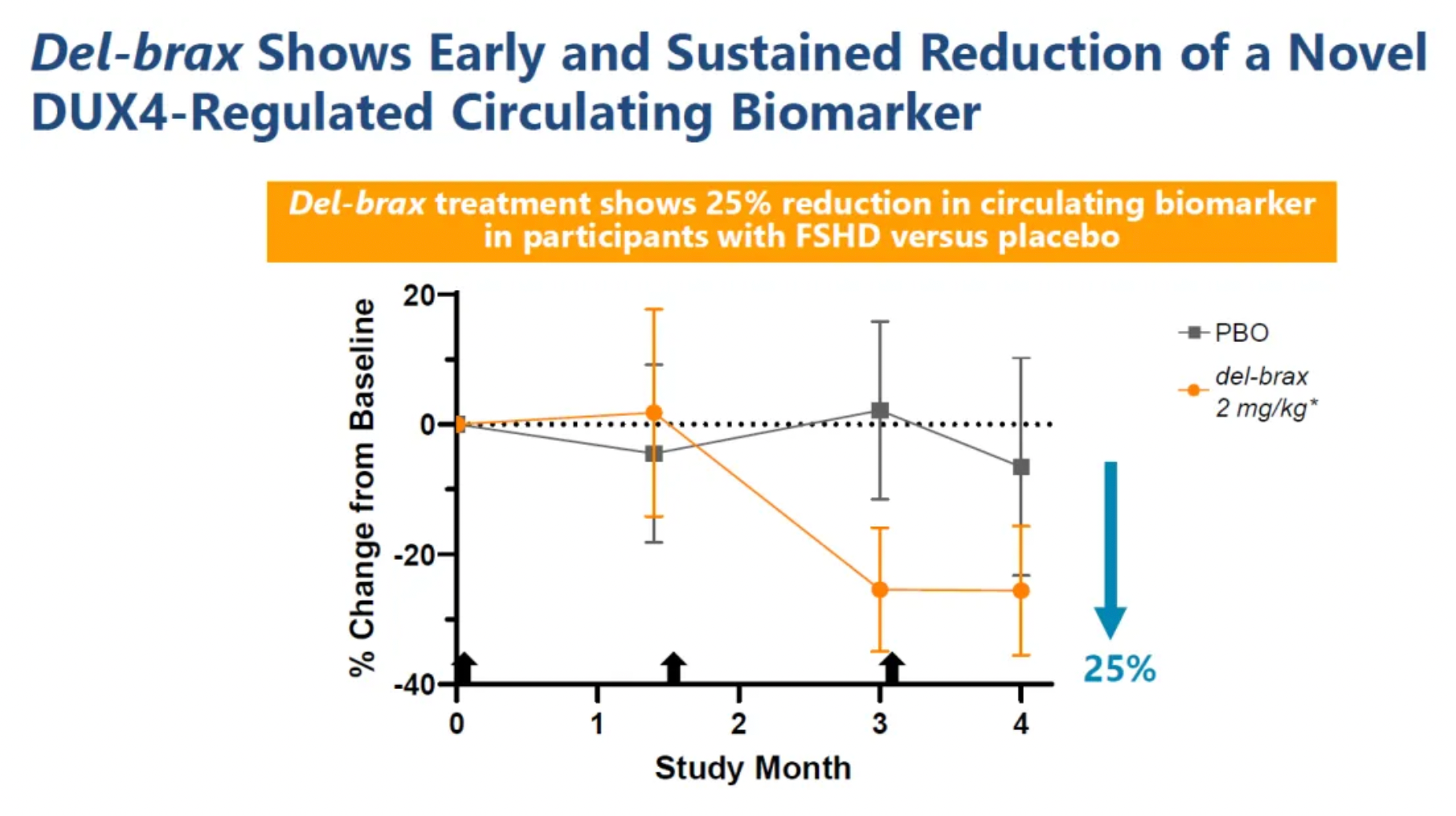
As illustrated below, in the four-month FORTITUDE evaluation, patients in the 2 mg/kg cohort received a single dose of 1 mg/kg AOC 1020 followed by two doses of 2 mg/kg AOC 1020 (siRNA dosage) or placebo. In the 2 mg/kg cohort, all participants who received the study treatment showed a reduction in DUX4 regulatory genes greater than 20%. Additionally, researchers reported a new circulating biomarker and an average reduction of at least 25% in creatine kinase levels.
Antibody-oligonucleotide conjugates (AOCs) are a new class of synthetic chimeric biomolecules that have gained increasing attention in various fields of modern biotechnology. This attention is primarily due to the unique combination of properties of their two components, the exceptional targeting capabilities and biologic distribution profile of antibodies, and the extensive functional and structural roles of oligonucleotides. Combining these two biomolecules into a single chimeric structure has proven to be a significant milestone in the advancement of many biotechnological applications, including imaging (DNAPAINT), detection (PLA, PEA), and treatment (targeted siRNA/antisense delivery). Numerous synthetic methods have been developed to obtain AOCs, ranging from random biochemical conjugation to site-specific coupling using active handles introduced into the antibody sequence through protein engineering.

Creating chimeric structures by combining different functionalities within one entity has always been an attractive strategy in the fields of biology, chemistry, and medicine. From chemical functionalization of natural proteins to the creation of chimeric structures using recombinant protein methods, these approaches have resolved many technical and fundamental issues across diverse areas of modern research. For example, advances in recombinant technology have enabled the “fusion” of different protein functionalities within a single molecule, which in turn has led to the emergence of a new family of chimeric therapies. Although proteins exhibit a wide range of functionalities, this diversity can be further expanded by introducing non-protein tags (also referred to as labels). For instance, by conjugating proteins with fluorescent markers or cytotoxic drugs, highly sensitive detection techniques and targeted therapeutics can be developed. In contrast to fusion proteins, where the stoichiometry of chemistry is tightly controlled, conjugation often results in mixtures of species with varying numbers of labels per biomolecule. Strict control of stoichiometry can greatly enhance many applications of protein conjugates, hence, there has been extensive research into new selective conjugation techniques.
Monoclonal antibodies and oligonucleotides (ONs) are two significant families of biomolecules that have garnered considerable attention and have utterly transformed the fields of therapy, diagnosis, and biology. Although these two families have each contributed to the development of many important therapeutic drugs and diagnostic technologies, chimeric entities did not begin to emerge until the 1980s.
Antibodies: Antibodies (Ab), also known as immunoglobulins (Ig), are depicted as Y-shaped glycoproteins produced by the immune system in B cells to protect organisms from pathogens such as bacteria and viruses. These large molecules (approximately 150 kDa and above) can recognize specific regions on antigens (usually foreign substances that trigger immune responses) and bind to them with high selectivity. Using this binding, antibodies can tag pathogens for further attack by different components of the immune system or directly neutralize the invaders by blocking mechanisms essential for the pathogen’s survival.
This precise antigen recognition and the unique functional characteristics of antibodies have ultimately led to their development as effective targeted therapeutic drugs, serving either as standalone treatments through antibody-dependent cell-mediated cytotoxicity (ADCC), or as vehicles for drug delivery (antibody-drug conjugates, ADCs) and imaging (immunofluorescence).
Oligonucleotides: Short RNA and DNA molecules, along with their synthetic alternatives (known as xeno nucleic acids, or XNAs), have been actively researched since the discovery of DNA's structure in 1953. Subsequently discovered biological mechanisms related to neural networks have spurred numerous applications. For many therapeutic applications, XNAs experiments need to overcome the fundamental limitations of natural RNA and DNA substrates, such as by enhancing their stability in vivo.
With the advancement of solid-phase chemical synthesis technology, the extensive application of nucleotides has become possible. To date, they have become key components of many common laboratory techniques, including Polymerase Chain Reaction (PCR), DNA sequencing, and array preparation. Recently, the first therapeutic application has also become possible and has been increasingly popularized to this day. Since the discovery of siRNA, hundreds of clinical trials have been conducted, and in 2018, the first drug, patisiran (Onpattro), was approved for marketing.
A critical parameter of nucleotides is their length (number of nucleotide bases, denoted by the suffix -mer) and composition. These two parameters define much of ON's characteristic and functional properties. Thus, ribonucleic acids can perform many functional roles, the most important of which include structural roles (DNA folding), targeting (aptamers), detection (DNA microarrays), regulation of gene expression (microRNA, miRNA), and gene silencing (antisense RNA, ASO, and small interfering RNA, siRNA). Finally, ON's selective Watson-Crick base pairing properties make them perfect platforms for assembling various molecular structures.
Antibody and oligonucleotide conjugation: Therefore, antibodies and ON molecules have proven to be powerful tools in analysis, detection, and therapeutic applications across various fields. Combining these different functionalities in a chimeric structure has only recently been explored and has become of interest in both fundamental research and industrial applications.
To understand the growing demand for AOCs, it is essential to recognize the unique strengths and functionalities of these two components: the high affinity of antibodies (disassociation constant in the sub-nanomolar range) and the versatility of ON functionalities. Due to this diversity of functionality, these structures can trigger complex biological mechanisms, ranging from immune responses to cell differentiation, apoptosis, and protein expression.
In AOC applications, antibodies are typically used as the target recognition unit, while ONs fulfill multiple functional roles, such as sensitive signal amplification (PCR), serving as scaffolds for spatial arrangement, or as therapeutic agents (siRNA). In the case of therapeutic agents (Ab-siRNA), antibodies can serve as delivery vehicles by increasing the circulating time of therapeutic agents in vivo and by limiting their renal elimination due to the larger size of the resulting chimeric structure.
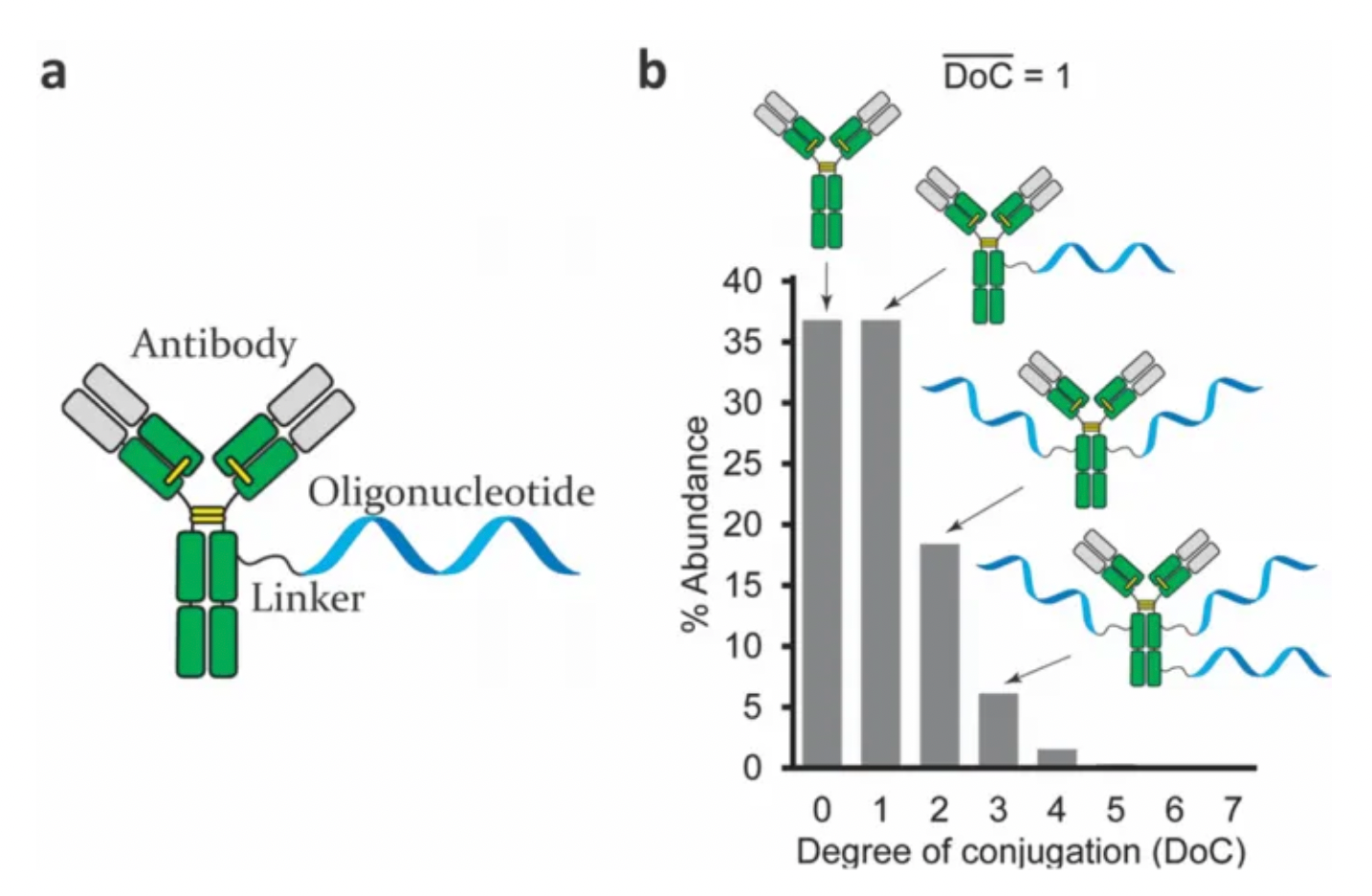
One key characteristic of bioconjugates is the degree of conjugation (DoC), indicating the number of ON molecules per antibody. Average DoC values can be determined by mass spectrometry or gel electrophoresis. DoC directly determines the molecular weight and size of the AOC, thereby affecting its physicochemical properties such as solubility, affinity, and aggregation. Moreover, the significant negative charge of the ONs has a critical impact on antibodies, which are positively charged at neutral pH. Hence, the total charge of the conjugate material is proportional to the DoC. In terms of analytical methods, during mass spectrometry, DoC can affect the conjugates' sensitivity to ionization, leading to variable peak ratios among conjugates with different DoC values. This brings considerable challenges to the precise and reliable quantitative analysis of AOC compositions, necessitating new analytical techniques.
In therapeutic applications, Antibody-Oligonucleotide Conjugates (AOCs) have been used as therapeutic agents containing ASOs or siRNAs as payloads, and as pre-targeting modules for radioisotope therapy and imaging. In these instances, the antibody provides targeting to the site of interest, where the attached oligonucleotide (ON) can exert its function, serving as a gene silencing agent or a pre-targeting therapeutic.
Due to their significant role in gene regulation, gene silencing, protein synthesis, and enzyme inhibition, numerous therapeutic applications have been proposed based on ONs functioning as pre-targeting agents or therapeutics. As a therapeutic approach, AOCs have been reported as promising agents for gene-silencing anti-cancer and antiviral drugs, radiotherapy enhancers, or as tools for studying antibody internalization and metabolic mechanisms.
From a manufacturing perspective, most therapeutic AOCs are obtained via recombinant expression of targeting fragments fused with protamine. Protamine is a verified cationic peptide used for non-covalent ion complexing. Many covalent AOCs have been created chemically, including the introduction of maleimide and azide residues into mAbs, followed by reaction with thiol or alkyne-modified ONs, respectively. An effective release mechanism that allows ONs to enter the cytoplasm (without being recycled to the extracellular space or transported to lysosomes) remains a stumbling block in the ongoing development of AOCs as therapeutic drugs.
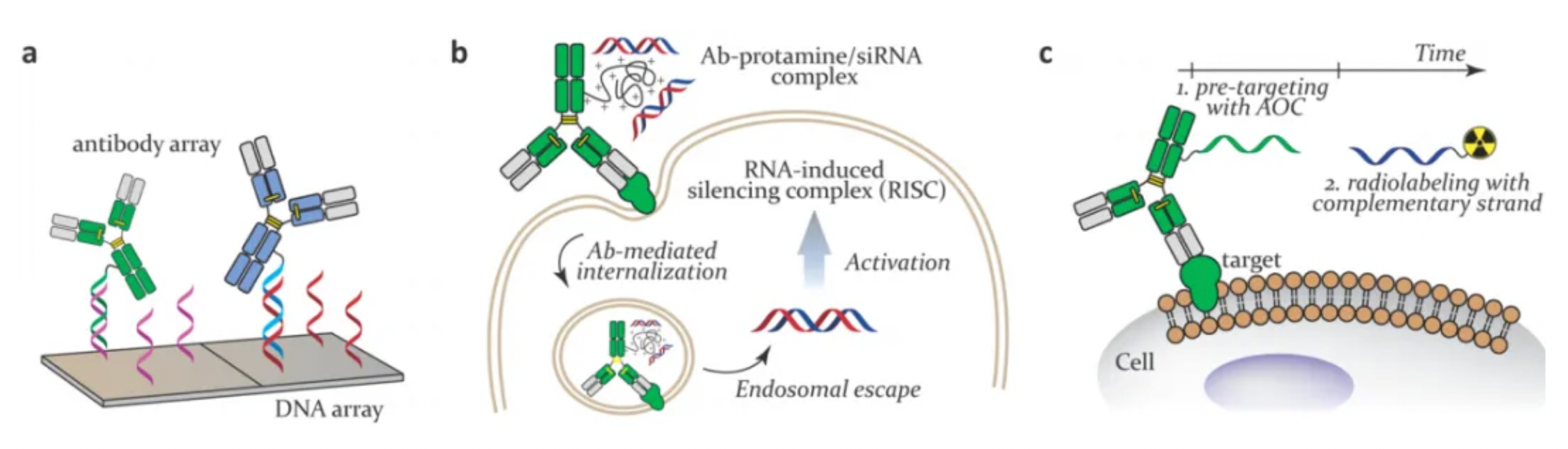
Typically, prolonged AOC (Antibody-Oligonucleotide Conjugate) circulation times can be problematic in therapies based on potent cytotoxic agents. For example, in radioimmunotherapy, adopting a two-step treatment protocol can minimize the impact on normal tissues. In this approach, a "naked" AOC targeting a specific marker is first administered, allowing it to be absorbed and distributed (pre-targeting). Following this step, the administration of a radioactive isotope-labeled complementary oligonucleotide (ON), which distributes more rapidly, results in a more favorable uptake ratio of tumor versus normal tissue than directly administering radioisotope-labeled antibody conjugates.
Summary
The coupling of biomolecules with distinct functions within a single chimeric structure has achieved substantial success in various applications. Many of the latest advances in biotechnology, such as novel therapeutic approaches (like fusion proteins, gene therapy, ADCs) and new sensitive detection methods (like iPCR), are the result of these efforts.
The conjugation of two distinctly different biomolecules - antibodies, known for their specific targeting capabilities, and oligonucleotides, known for their rich functionality, structure, and signaling capabilities - has recently become an active and consistent focus in bioconjugation research. Given the proven functional potential of these two fragments and the rapid development of biological and chemical tools for bioconjugation, AOCs are set to play a significant role in modern medicine, biology, and chemistry soon.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
References
1.https://www.neurologylive.com/view/aoc-1020-shows-significant-impact-underlying-pathology-facioscapulohumeral-muscular-dystrophy.
2.Alain Wagner et al, Antibody−Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjugate Chem,DOI: 10.1021/acs.bioconjchem.9b00306.