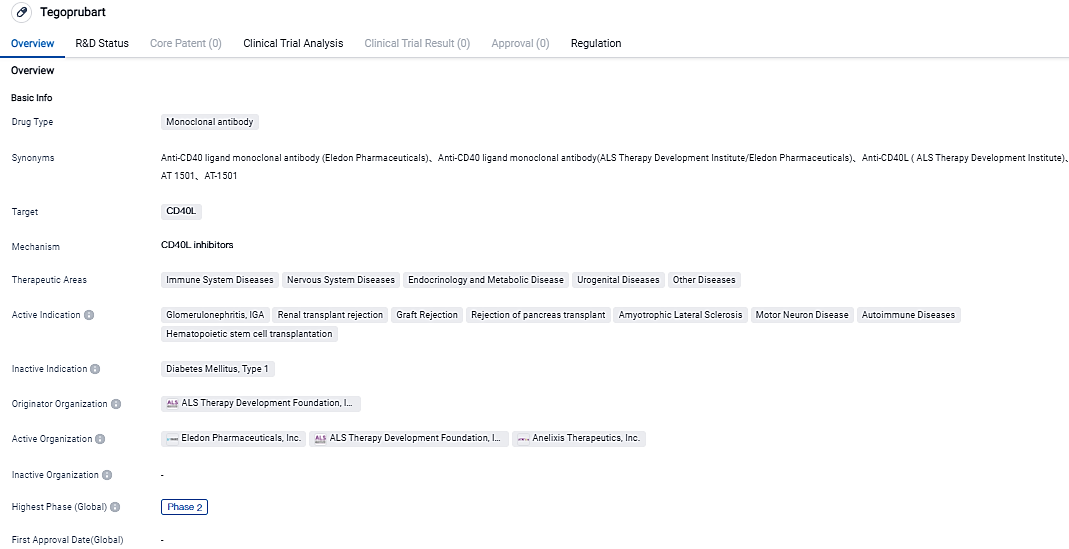
Eledon Pharmaceuticals reports first participant dosed in Phase 2 BESTOW study, assessing Tegoprubart against rejection in kidney transplants
Eledon Pharmaceuticals, Inc. revealed that it has administered the initial dose to the first participant in the BESTOW Phase 2 study. This trial is directed at assessing tegoprubart for preventing organ rejection in patients receiving kidney transplant.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The ESTOW, a large-scale, bi-directional, active reference clinical investigation, intends to include approximately 120 individuals undergoing renal transplantation in the United States and other nations. This is aimed at assessing the safety, pharmacokinetics, and effectiveness of the anti-CD40 ligand antibody tegoprubart, in contrast to the calcineurin inhibitor known as tacrolimus.
The primary aim of this research is to evaluate graft operational abilities at 12 months post-transplantation, gauged by the estimated glomerular filtration rate in individuals treated with tegoprubart against those treated with tacrolimus. Enhanced graft functioning, as determined by eGFR, has been linked with enhanced long-term survival rates for patients and grafts.
Secondary objectives will encompass graft survival, biopsy-confirmed acute rejection, and the incidence of new onset diabetes mellitus following transplantation. Eledon will also employ the iBox Scoring System - a combined endpoint of renal graft functioning utilizing clinical, histological, and serum biomarkers - as a tool for the premature prediction of graft failure. This will be used as an investigative endpoint.
"We are thrilled to commence enrollment for the Phase 2 BESTOW clinical trial, which is an imperative subsequent phase in our evaluation of tegoprubart," proclaimed David-Alexandre C. Gros, M.D., the CEO of Eledon. "We anticipate gaining further understanding into the therapeutic potential of tegoprubart compared to standard care, all the while running our Phase 1b in parallel. This positions us for reporting numerous data updates over the following 18-month period." Eledon has previously published outcome findings for the first three individuals administered in the Company’s ongoing Phase 1b trial. The results exhibited no occurrences of acute rejection and solid graft operating capacity noted in all three subjects. The trial has currently enlisted 11 individuals so far and the operations will proceed in tandem with the Phase 2 BESTOW trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
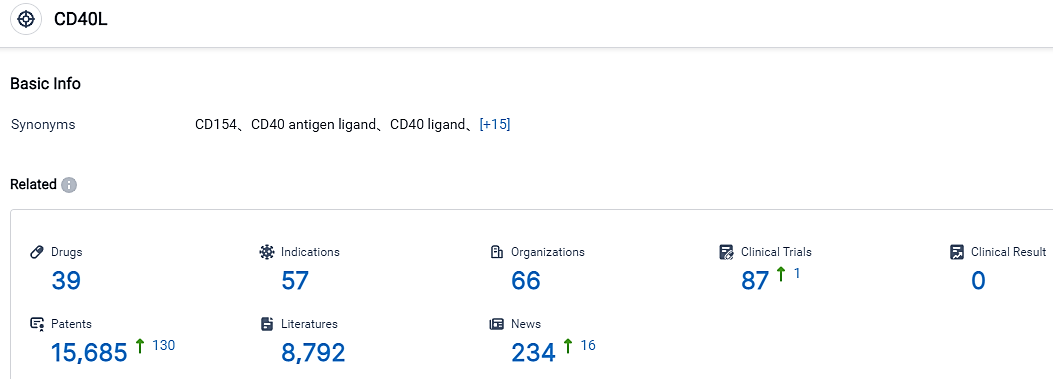
According to the data provided by the Synapse Database, As of September 7, 2023, there are 39 investigational drugs for the CD40L target, including 57 applicable indications,66 R&D institutions involved, with related clinical trials reaching 87,and as many as 15685 patents.
The therapeutic benefits of Tegoprubart seem promising for a variety of diseases and conditions, particularly those involving the immune and nervous systems, as well as transplantation. Tegoprubart interacts specifically with CD40L, a protein that plays a significant role in immune activations, which could possibly enhance its healing properties. Additional investigative studies and improvements, including moving forward to more advanced stages of clinical experiments and securing regulatory consent, will be required to ascertain the final efficacy and market viability of the drug.