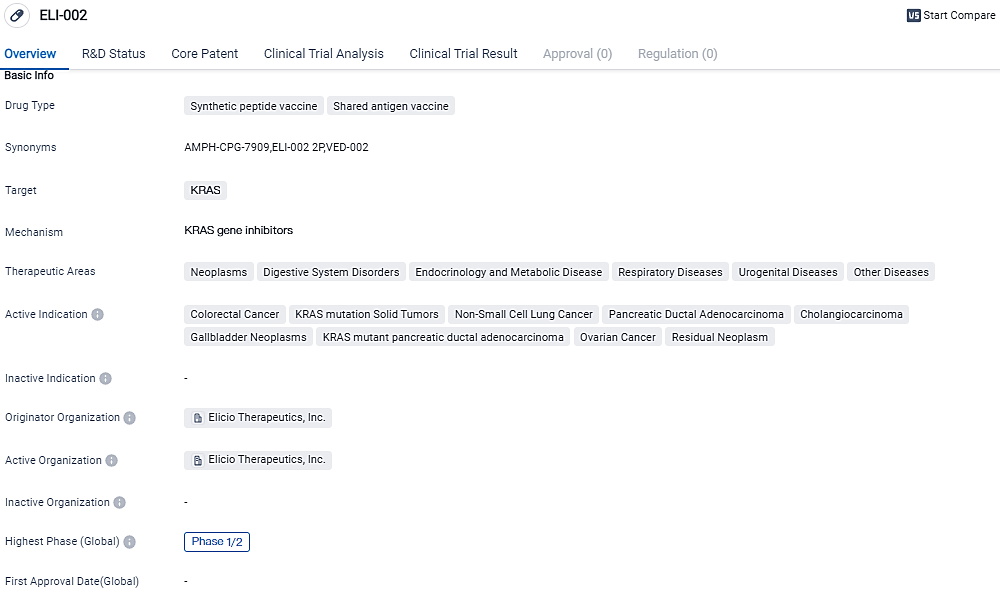
Elicio Therapeutics Showcases Findings from the initial Phase of the ELI-002 Study
Elicio Therapeutics, Inc., a biotechnology firm in the clinical trial phase with a focus on creating groundbreaking immunotherapies for cancer treatment, has revealed encouraging initial data related to relapse-free survival from their ongoing Phase 1 investigation of their primary asset, ELI-002.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The investigation of ELI-002 2P, an engineered 2-peptide drug aimed at treating G12D and G12R KRAS mutation-triggered cancers, as a standalone treatment for patients with solid tumors linked to mutant KRAS, was the focus of this research.
“Patients diagnosed with KRAS mutant cancers, especially those with pancreatic and colorectal cancers, often face bleak chances of recovery and limited therapeutic strategies when tumor DNA or protein biomarkers are identified post-standard surgical procedures and chemotherapy. The preliminary data, suggesting that ELI-002-induced T cells may positively affect clinical evaluation metrics including relapse and mortality risks, offers us hope," stated Eileen M. O’Reilly, M.D., Winthrop Rockefeller Endowed Chair of Medical Oncology; Co-Director.
Christopher Haqq, M.D., Ph.D., Elicio’s Primary Vice President, Research and Development Head, and Senior Medical Officer, stated, "The findings strengthen the understanding of ELI-002's mode of operation and the probable advantage of delivering the cancer vaccine to lymph nodes. ELI-002 provokes an effective mKRAS-specific T cell response, including CD4 and CD8 categories, which appears to correlate with tumor biomarker responses, and the findings hint this might enhance clinical outcomes."
Christopher Haqq, M.D. added, "The initial findings give us optimism and we anticipate remaining engaged in tracking and disseminating more clinical and biomarker data concerning the AMPLIFY-201 study. Moreover, the independent data tracking committee of the AMPLIFY-7P study encouraged the launch of a phase 2 trial evaluating ELI-002 7P as a single therapy in adjuvant PDAC patients."
ELI-002 is a structurally innovative exploratory AMP immunotherapy that targets cancers caused by mutant KRAS. KRAS mutations rank among the most frequently encountered human cancers. ELI-002 includes AMP-modified mutant KRAS peptide antigens and ELI-004, an AMP-modified immune-stimulating oligonucleotide CpG adjuvant. The AMP mKRAS peptides and AMP CpG are routed towards the lymph node where they might potentially augment the action of crucial immune cells.
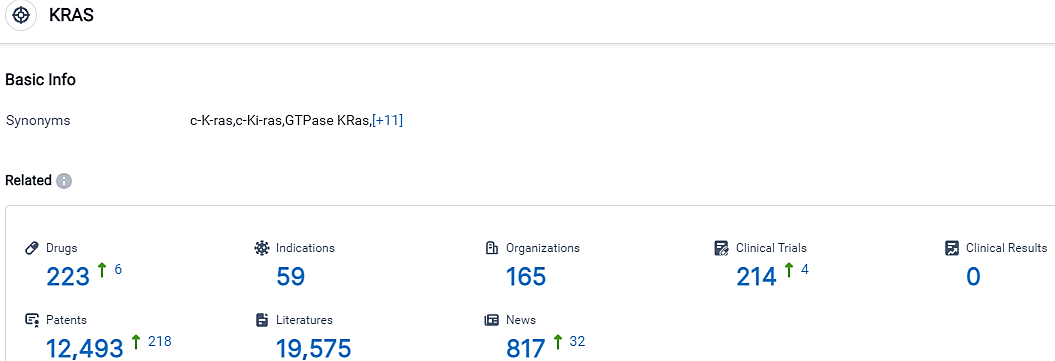
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 7, 2023, there are 223 investigational drugs for the KRAS target, including 59 indications,165 R&D institutions involved, with related clinical trials reaching 214,and as many as 12493 patents.
ELI-002 2P is currently being studied in a Phase 1 trial in patients with high relapse risk mKRAS-driven solid tumors, following surgery and chemotherapy. The ELI-002 7P formulation is designed to provide immune response coverage against seven of the most common KRAS mutations, thereby increasing the potential patient population for ELI-002 and potentially reducing the chance of bypass resistance mechanisms.