Harpoon Therapeutics Unveils Initial Phase 1 Results of HPN217 at IMS Yearly Conference
Harpoon Therapeutics, Inc., a company in the clinical-phase focusing on creating new T cell engagers for immunotherapy, has revealed the interim results pertaining to the target dose groups ranging from 2.15 to 12 mg from its Phase 1 clinical examination. This was carried out to assess the single-agent HPN217's efficacy in treating patients with relapsed or refractory multiple myeloma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
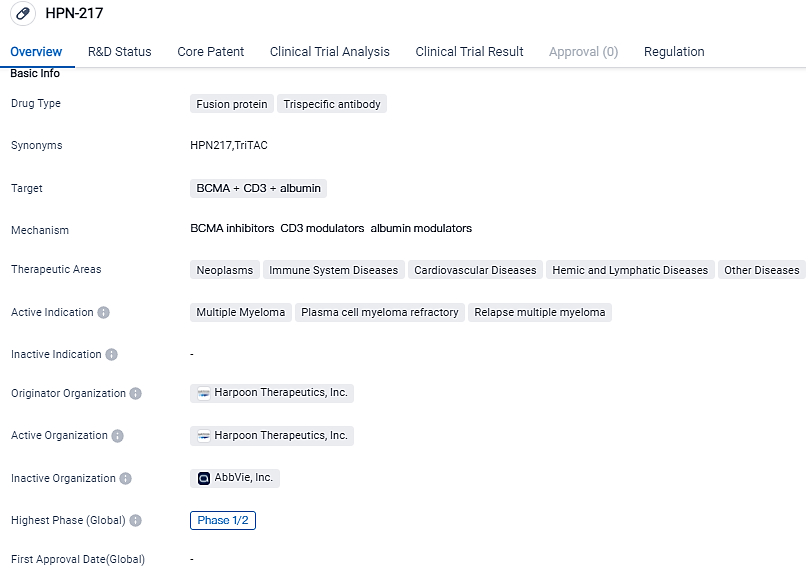
HPN217 is aimed at the B-cell maturation antigen (BCMA) and is built upon Harpoon's own Tri-specific T cell Activating Construct (TriTAC®) platform, designed to mobilize a patient’s immune cells to annihilate tumor cells. These results will be showcased in a poster presentation at the 20th International Myeloma Society Annual Meeting in Athens, Greece, on September 28, 2023.
As per the data cut-off as of August 12, 2023, HPN217 had been administered to 97 patients across 14 dose-increasing groups. The participants in the trial were intensely treated before, with 65% exposed to five drugs, and 20% previously treated with BCMA-related drugs.
Patients at the 12 mg target dose displayed manageable side effects and low incidences of CRS and zero of ICANS. Plus, promising initial clinical results were observed. Seven responders are still receiving treatment, while the median response duration is currently extending to 8.3 months. Among the initial 2.15mg to 6mg dose groups, the median response duration was 21.8 months, with several patients continuing treatment and maintaining a response.
Luke Walker, M.D., the Chief Medical Officer for Harpoon Therapeutics, commented notably on the early and lasting responses of the 12 mg group of HPN217 in a RRMM patient group previously treated extensively, '' The 63% overall response rate at the target dose is stout, with many responses happening early and deepening as time devolves." He added, "The rarity of CRS and impressive efficacy further validate the advanced development of HPN217 and its prospective emergence as a distinguished choice for patients."
In terms of enrolment in escalating dose groups up to 24 mg, it's complete, with the maximum tolerable dose yet to be established. Follow-ups are constant, and the evaluation of protocols will guide the selection of an advised Phase 2 dose or doses by the end of 2023.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
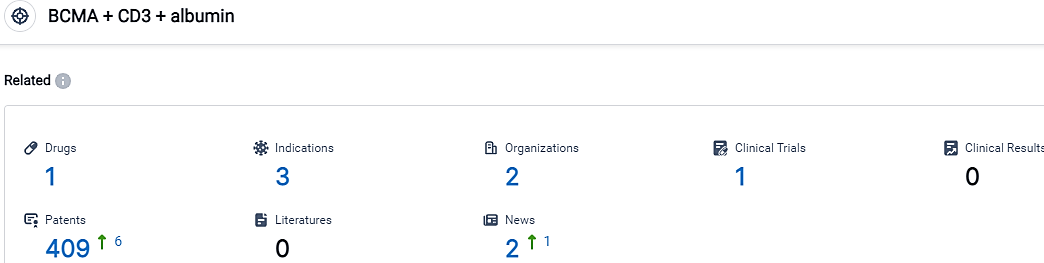
According to the data provided by the Synapse Database, As of October 5, 2023, there are 1 investigational drugs for the BCMA and CD3 and albumin target, including 3 indications,2 R&D institutions involved, with related clinical trials reaching 1,and as many as 409 patents.
Harpoon Therapeutics, Inc. is the developer of HPN-217, a trispecific antibody and fusion protein medication. This particular drug is designed to target BCMA, CD3, and albumin, and its development and potential use in the treatment of multiple myeloma and other diseases is currently under investigation. Presently, HPN-217 is in the Phase 1/2 stage of clinical trials and has received both Fast Track and Orphan Drug approvals.