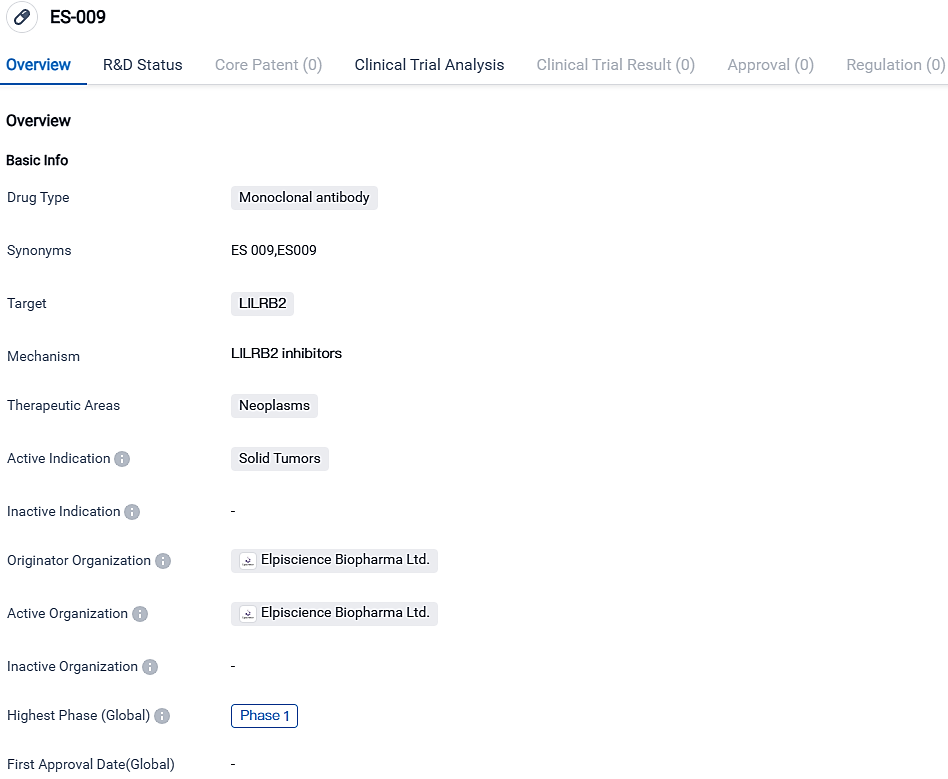
Elpiscience initiates Phase 1 clinical trial of Anti-LILRB2 Antibody ES009 with first patient dosed in Australia
Elpiscience Biopharmaceuticals, a progressive biopharmaceutical entity focusing on the innovation and development of future cancer immunotherapies, has revealed that the initial patient has received a dose in a Phase 1 clinical study of the anti-LILRB2 monoclonal antibody ES009 in Australia. The primary aim of the study is to assess its safety, tolerability, pharmacokinetics, pharmacodynamics, along with early clinical efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Notably known as ILT4, LILRB2 is an essential inhibitory receptor predominantly found on myeloid cells, playing a significant role in immune suppression within tumor microenvironments. ES009 is specially designed to interact with a unique epitope on human LILRB2, proving successful in inhibiting LILRB2's binding to numerous ligands.
ES009 effectively negates LILRB2-mediated inhibitory signals, successfully transforming myeloid cells from an anti-inflammatory to a pro-inflammatory phenotype, while also bolstering the functions of T cells. In preclinical trials, ES009 has shown incredible potential in overturning immune suppression in the TME and fostering anti-tumor immunity.
LILRB2 is a crucial immune checkpoint in the realm of tumor immunotherapy, its inhibitory influence on the immune response has been established as a contributing factor to anti-PD(L)1 resistance. We are deeply committed to innovating treatments for cancer patients that are resistant or non-responsive to PD(L)1 treatment.
"We created ES009, which has rapidly emerged as the leading contender in its class. We have faith in the potential of innate immunity enhancement as a strategy against cancer and have developed a distinctly myeloid cell-centric therapeutic portfolio. We are passionately dedicated to delivering these promising therapies to cancer patients who have critical medical needs," shares Dr. Hongtao Lu, co-founder and Chief Scientific Officer of Elpiscience.
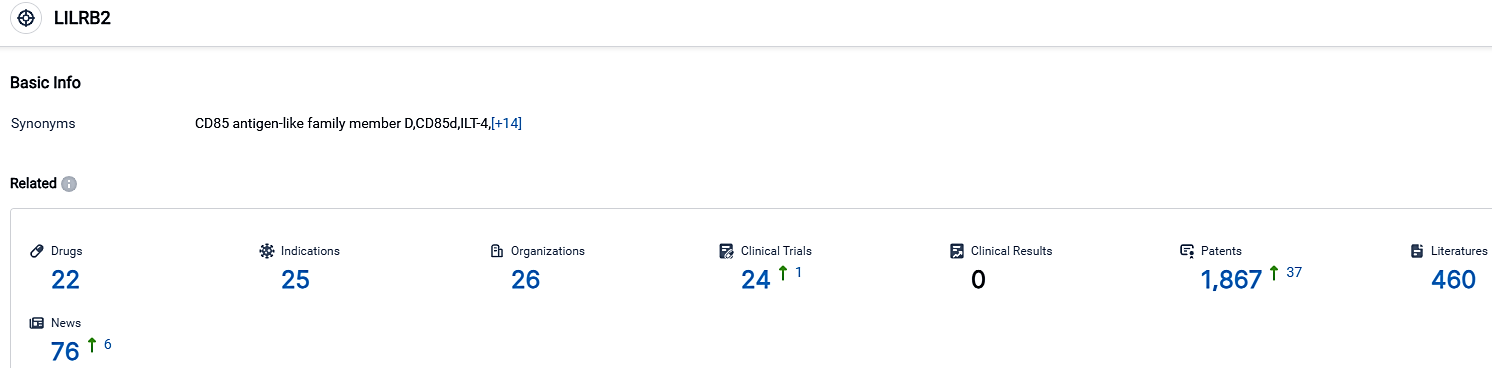
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 2, 2023, there are 22 investigational drugs for the LILRB2 target, including 25 indications, 26 R&D institutions involved, with related clinical trials reaching 24,and as many as 1867 patents.
ES-009 exhibits potential as a therapeutic approach for solid tumors. Nevertheless, additional clinical examinations and investigations must be conducted to ascertain its safety and efficacy in human beings. The present progression state of the drug indicates that it is in the preliminary phases of development and possibly more time and resources are required for approval for patient treatment.