EMA Accepts ENHERTU® Application for Treatment of HER2 Low/Ultralow Metastatic Breast Cancer Post-Endocrine Therapy
Daiichi Sankyo (TSE: 4568) revealed that the European Medicines Agency (EMA) has acknowledged the Type II Variation submission for ENHERTU® (trastuzumab deruxtecan) as a standalone treatment for adult patients with unresectable or metastatic HER2 low (classified as IHC 1+ or IHC 2+/ISH-) or HER2 ultralow (classified as IHC 0 with membrane staining) breast cancer who have undergone a minimum of one endocrine therapy in the metastatic context.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ENHERTU is a specifically engineered HER2-directed DXd antibody-drug conjugate (ADC) discovered by Daiichi Sankyo, and it is being developed and marketed collaboratively by Daiichi Sankyo and AstraZeneca (LSE/STO/Nasdaq: AZN).
Validation confirms that the submission is comprehensive, initiating the scientific evaluation process by the EMA’s Committee for Medicinal Products for Human Use. This submission is supported by data from the DESTINY-Breast06 phase 3 trial, which was presented during a late-breaking oral session at the 2024 American Society of Clinical Oncology (#ASCO24) Annual Meeting.
“This application builds on the current indication for ENHERTU in patients with HER2-low metastatic breast cancer, and an expanded approval could allow for its potential use in earlier stages of the disease and in a broader patient population, including those with HER2-ultralow,” stated Ken Takeshita, MD, Global Head of R&D at Daiichi Sankyo. “We eagerly anticipate collaborating with the EMA to potentially make this medicine available to more patients in the EU.”
Additional regulatory submissions for ENHERTU in this indication are in process globally.
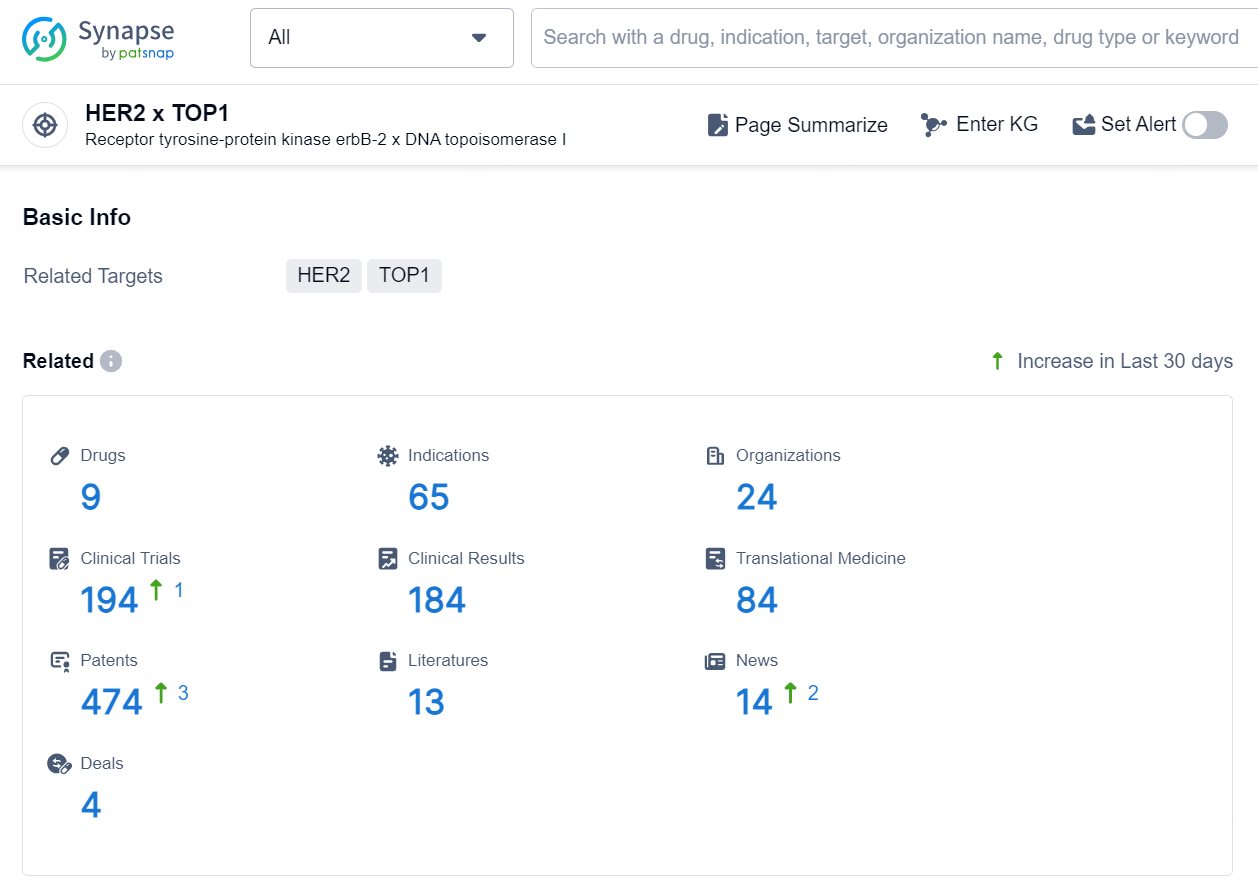
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
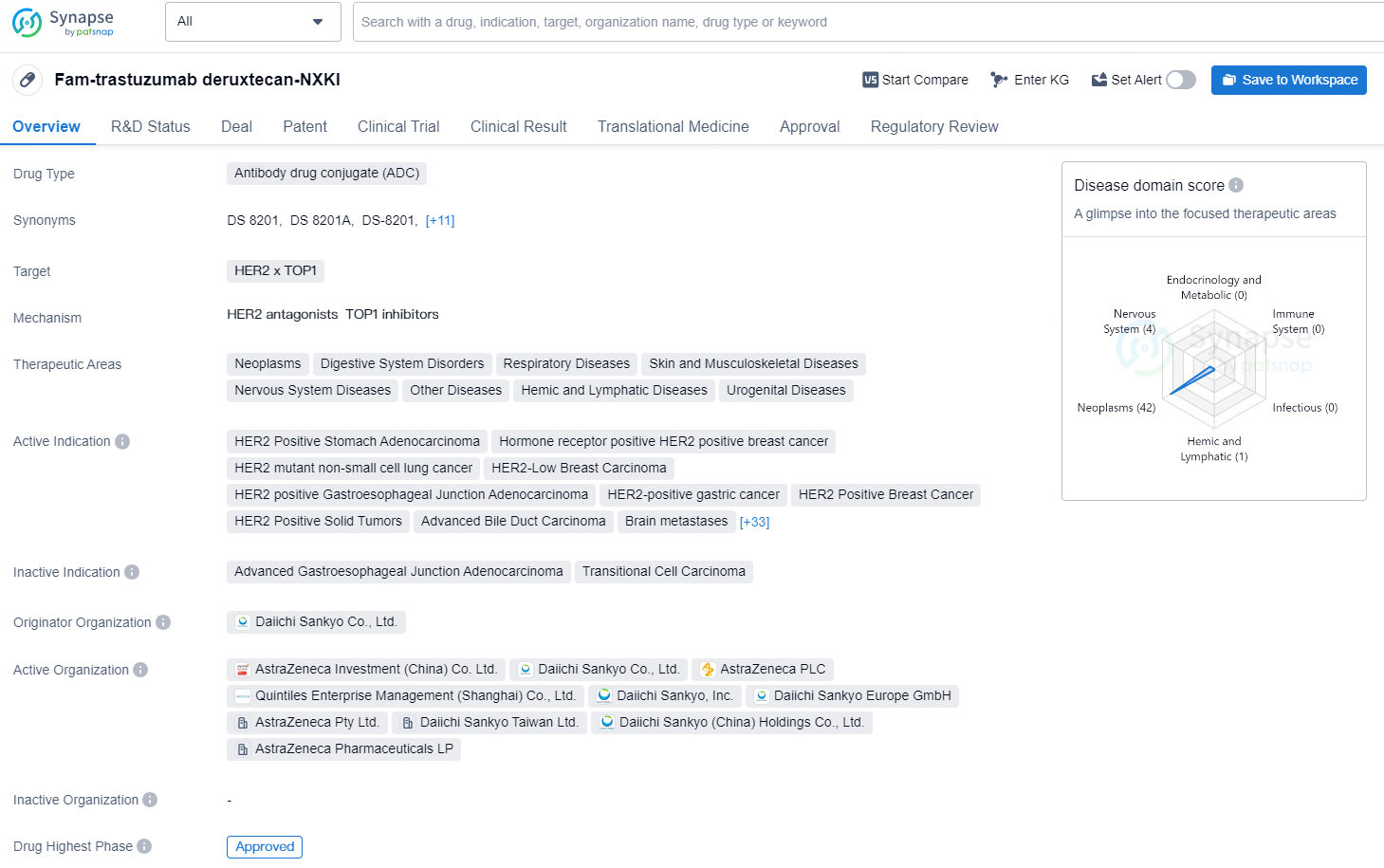
According to the data provided by the Synapse Database, As of August 21, 2024, there are 9 investigational drugs for the HER2 x TOP1 target, including 65 indications, 24 R&D institutions involved, with related clinical trials reaching 194, and as many as 474 patents.
Fam-trastuzumab deruxtecan-NXKI is an antibody-drug conjugate (ADC) medication developed by Daiichi Sankyo Co., Ltd. The drug targets HER2 and TOP1 and is indicated for a wide range of therapeutic areas, including neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, nervous system diseases, hemic and lymphatic diseases, and urogenital diseases..