Imfinzi Granted Priority Review and Breakthrough Therapy Status for Limited-Stage Small Cell Lung Cancer in the US
AstraZeneca's additional Biologics License Application (sBLA) for Imfinzi (durvalumab) has been accepted and given Priority Review status in the United States. This decision is based on favorable data from the ADRIATIC Phase III study involving patients with limited-stage small cell lung cancer (LS-SCLC) whose condition remained stable following platinum-based concurrent chemoradiotherapy (cCRT).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The Food and Drug Administration (FDA) provides Priority Review status to drug applications that propose treatments potentially offering considerable improvements over current options by enhancing safety or effectiveness, preventing severe conditions, or improving patient adherence. The FDA action date for their regulatory decision under the Prescription Drug User Fee Act is projected for the fourth quarter of 2024.
The Food and Drug Administration (FDA) provides Priority Review status to drug applications that propose treatments potentially offering considerable improvements over current options by enhancing safety or effectiveness, preventing severe conditions, or improving patient adherence. The FDA action date for their regulatory decision under the Prescription Drug User Fee Act is projected for the fourth quarter of 2024.
Imfinzi has also recently received Breakthrough Therapy Designation (BTD) from the FDA for this indication. BTD is intended to expedite the development and regulatory review of new medicinal products targeting serious conditions and addressing significant unmet medical needs.
Small cell lung cancer (SCLC) is an extremely aggressive variant of lung cancer that often recurs and advances rapidly, even after an initial favorable response to chemotherapy and radiotherapy in patients with limited-stage SCLC (LS-SCLC). The outlook for LS-SCLC is particularly dire, with only 15-30% of patients surviving five years post-diagnosis.
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, stated: “This Priority Review underscores the potential of Imfinzi to change patient outcomes as the first and only immunotherapy to show a survival advantage in limited-stage small cell lung cancer. There is a pressing need for new treatments that improve on the current standard of care, which has remained unchanged for forty years, and we are eager to collaborate with the FDA to make Imfinzi available to patients swiftly.”
The sBLA submission is supported by data from the ADRIATIC Phase III trial, which was recently showcased during the Plenary Session at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting.
In the trial, Imfinzi decreased the risk of death by 27% compared to placebo (based on an overall survival [OS] hazard ratio [HR] of 0.73; 95% confidence interval [CI] 0.57-0.93; p=0.0104). The median OS was 55.9 months for Imfinzi (95% CI 37.3-NE) versus 33.4 months for placebo (95% CI 25.5-39.9). It was estimated that 57% of patients receiving Imfinzi were alive at three years, compared to 48% of those on placebo.
Imfinzi also lowered the risk of disease progression or death by 24% (based on a progression-free survival [PFS] HR of 0.76; 95% CI 0.61-0.95; p=0.0161) compared with placebo. The median PFS was 16.6 months for Imfinzi (95% CI 10.2-28.2) versus 9.2 months for placebo (95% CI 7.4-12.9). It was estimated that 46% of patients treated with Imfinzi had not experienced disease progression at two years, in contrast to 34% on placebo.
The safety profile of Imfinzi was manageable and consistent with its known characteristics, with no new safety concerns identified.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
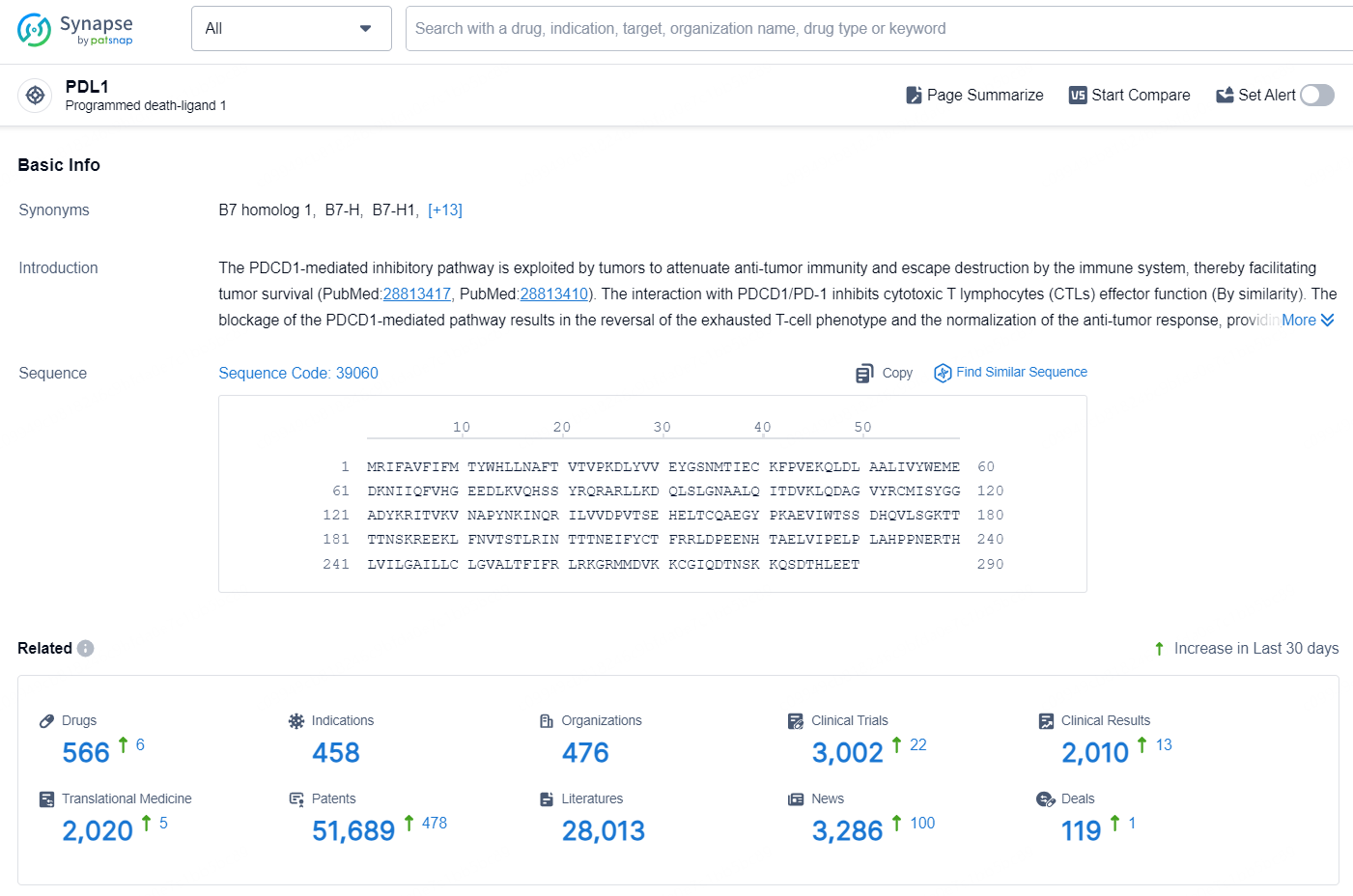
According to the data provided by the Synapse Database, As of August 20, 2024, there are 566 investigational drugs for the PDL1 targets, including 458 indications, 476 R&D institutions involved, with related clinical trials reaching 3002, and as many as 51689 patents.
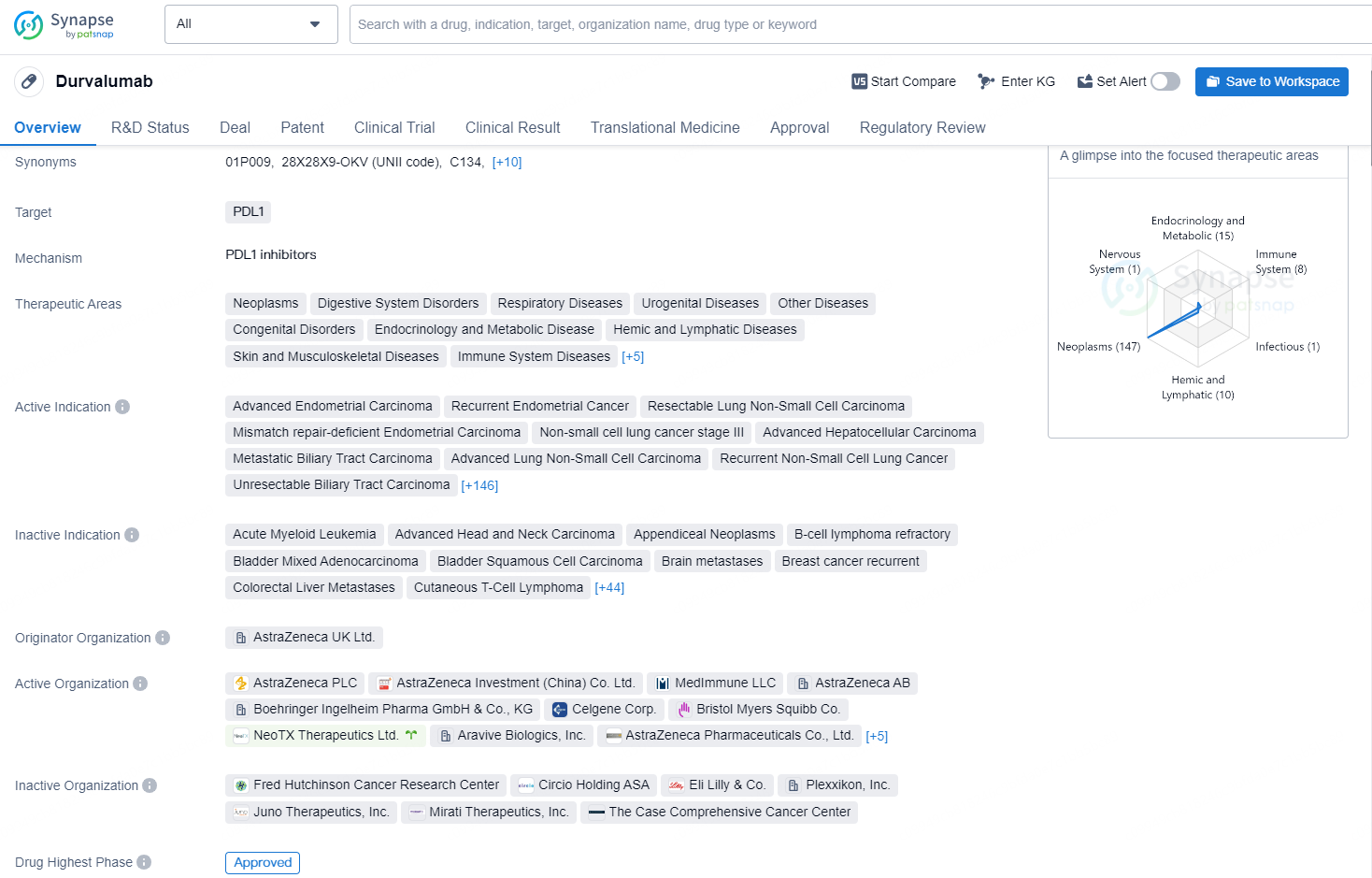
Imfinzi (durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumour’s immune-evading tactics and releasing the inhibition of immune responses. Imfinzi is the only approved immunotherapy and the global standard of care in the curative-intent setting of unresectable, Stage III NSCLC in patients whose disease has not progressed after chemoradiotherapy. Imfinzi in combination with chemotherapy (etoposide and either carboplatin or cisplatin) is also approved for the treatment of extensive-stage SCLC and in combination with a short course of Imjudo and chemotherapy for the treatment of metastatic NSCLC.