EMA Approves Henlius and Organon's Biosimilar HLX14 for Prolia® and Xgeva®
Shanghai Henlius Biotech, Inc. and Organon have announced that the European Medicines Agency has accepted the marketing authorization applications for HLX14, a biosimilar of the investigational drugs Prolia® and Xgeva® (denosumab).
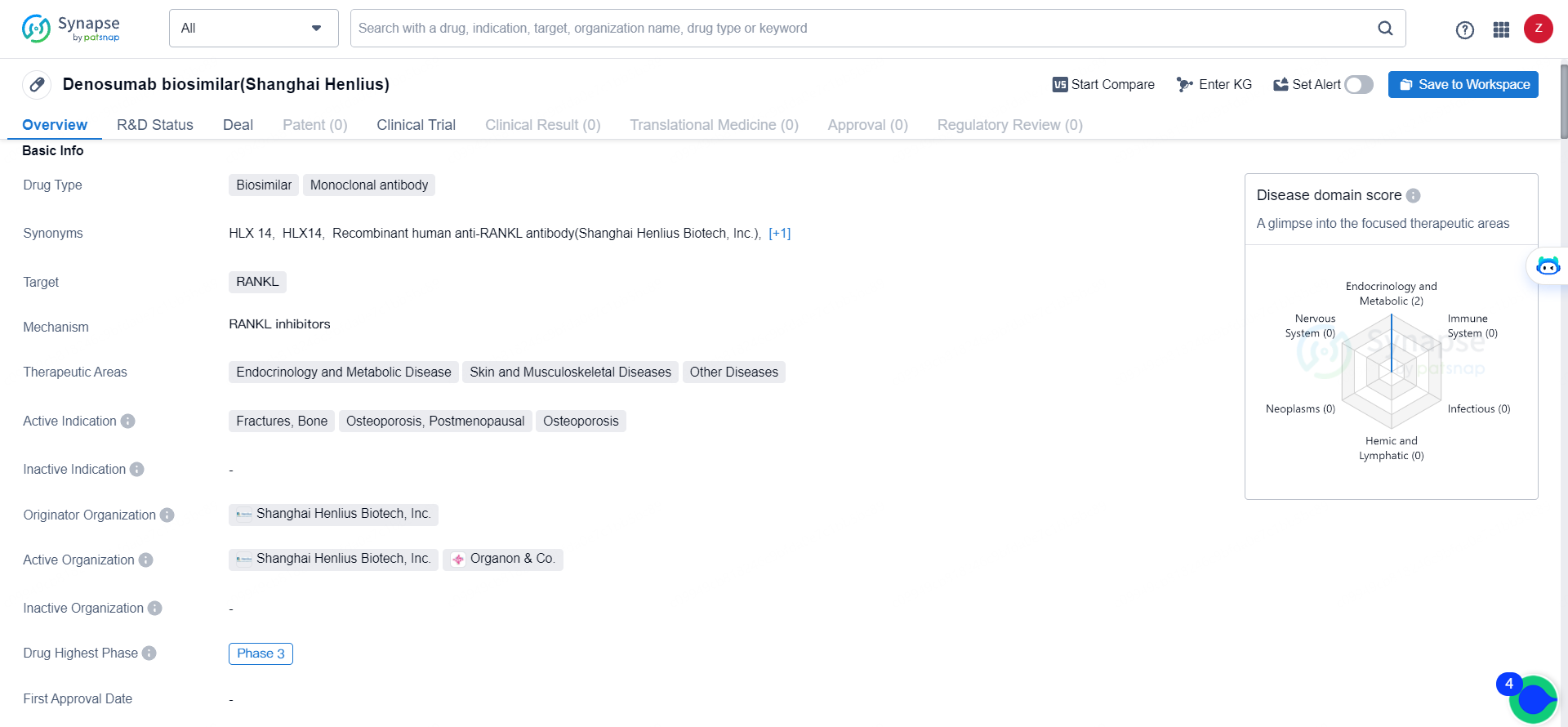
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Denosumab has received approval in multiple countries and regions under various trade names for different uses, including the treatment of osteoporosis in postmenopausal women at high risk of fractures. As of 2019, approximately 32 million Europeans aged 50 and above were estimated to have osteoporosis, with 25.5 million of them being women.
The approvals were based on data from a randomized, double-blind, international multicenter, parallel-controlled phase 3 clinical trial. This study aimed to evaluate the efficacy, safety, tolerability, and immunogenicity of HLX14 compared to EU-sourced reference denosumab in postmenopausal women with osteoporosis who are at high risk of fractures.
In 2022, Henlius signed a license and supply agreement with Organon, granting Organon the exclusive rights to commercialize two biosimilar candidates, including HLX14. This agreement covers regions such as the European Union, the United States, and Canada, but excludes China.
Henlius is a global biopharmaceutical company dedicated to providing high-quality, affordable, and innovative biologics for patients worldwide, with a focus on oncology, autoimmune diseases, and ophthalmic conditions. To date, they have launched 5 products in China, gained approval for 2 in international markets, received 23 global indications, and have 3 marketing applications under review in both China and the EU.
Since its establishment in 2010, Henlius has developed a comprehensive biopharmaceutical platform that integrates efficient and innovative capabilities throughout the entire product lifecycle, including R&D, manufacturing, and commercialization. They have set up a global innovation center and manufacturing facilities in Shanghai that comply with GMP standards in China, the EU, and the U.S.
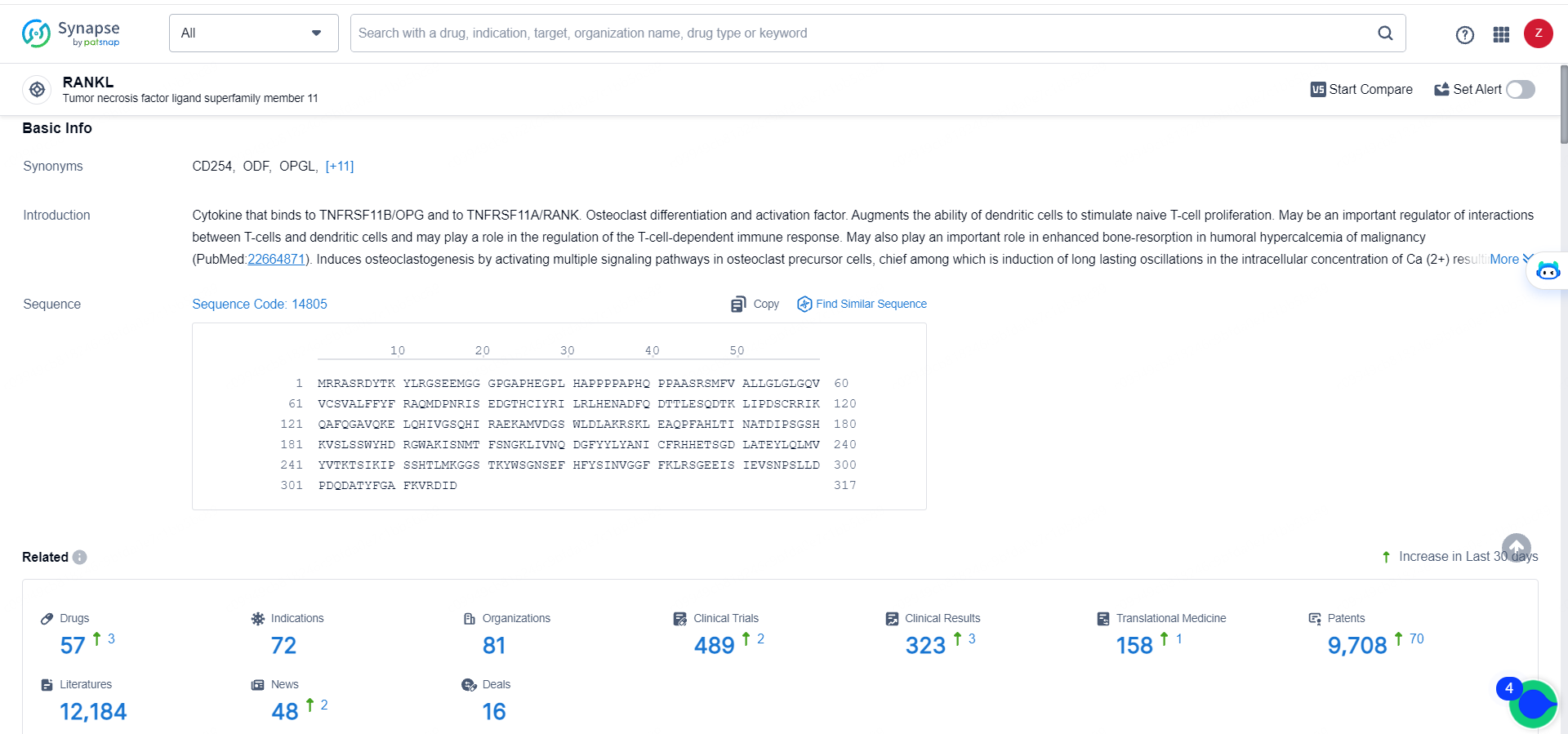
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 28, 2024, there are 57 investigational drugs for the RANKL targets, including 72 indications, 81 R&D institutions involved, with related clinical trials reaching 489, and as many as 9708 patents.
Denosumab aims to be highly similar to an existing biologic drug, offering a more affordable alternative while maintaining comparable efficacy and safety. By targeting RANKL, the drug is intended to address conditions related to bone health and metabolism, such as osteoporosis and fractures. The Phase 3 development stage suggests that the drug has shown promise in earlier clinical trials and is now being further evaluated for its effectiveness and safety in a larger patient population.