Zai Lab Starts Worldwide Phase 2 Study Testing ZL-1102 for Treating Chronic Plaque Psoriasis Topically
Zai Lab Limited revealed that the initial participant has received the dose in a worldwide Phase 2 clinical study assessing the effectiveness and safety of the company's proprietary anti-IL-17 experimental treatment, ZL-1102, aimed at treating chronic plaque psoriasis.
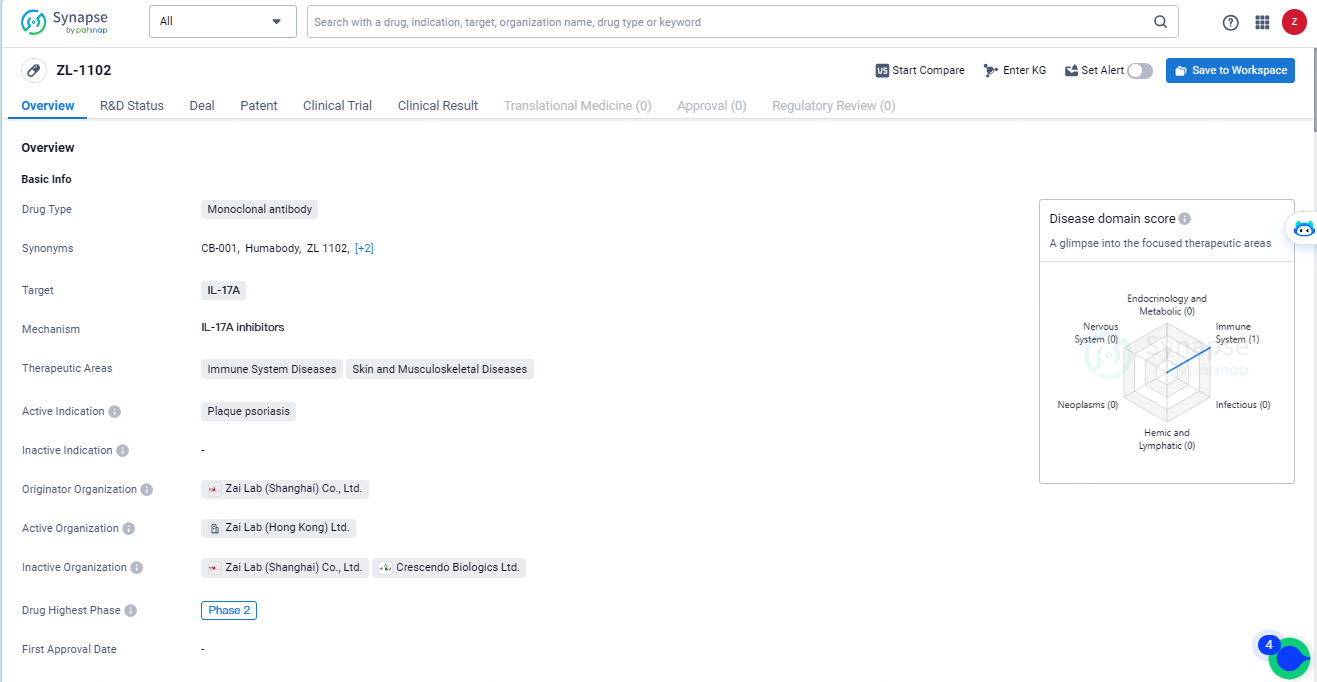
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ZL-1102 is an innovative human VH antibody fragment (Humabody®) specifically designed to bind to the IL-17 cytokine. Highlighting its distinct mechanism, ZL-1102 is being advanced as a topical therapy for mild-to-moderate chronic plaque psoriasis (CPP), setting it apart from other anti-IL-17 treatments that address moderate-to-severe cases through systemic delivery.
“This research represents a significant achievement in Zai Lab’s journey and showcases the dedication of our internal R&D team to create new therapies aimed at benefiting patients worldwide,” said Josh Smiley, President and Chief Operating Officer at Zai Lab.
“ZL-1102 is the pioneering IL-17-focused topical therapy under development for patients with milder CPP. By being designed for direct application on psoriatic skin plaques, we anticipate it can prevent unnecessary tissue exposure and reduce the systemic toxicity associated with intravenous or subcutaneous treatments,” said Dr. Harald Reinhart, President and Head of Global Development for Neuroscience, Autoimmune, and Infectious Diseases at Zai Lab.
ZL-1102, still under investigation, is a novel human VH antibody fragment targeting the IL-17A cytokine, formulated into a hydrogel for topical use in chronic plaque psoriasis treatment. Owing to its compact size and other distinctive properties of this molecule class, ZL-1102 exhibits enhanced target affinity and tissue penetration relative to full-sized monoclonal antibodies. This topical treatment could offer superior safety and tolerability, bringing the benefits of IL-17-targeted therapies to the broader patient population with less severe CPP.
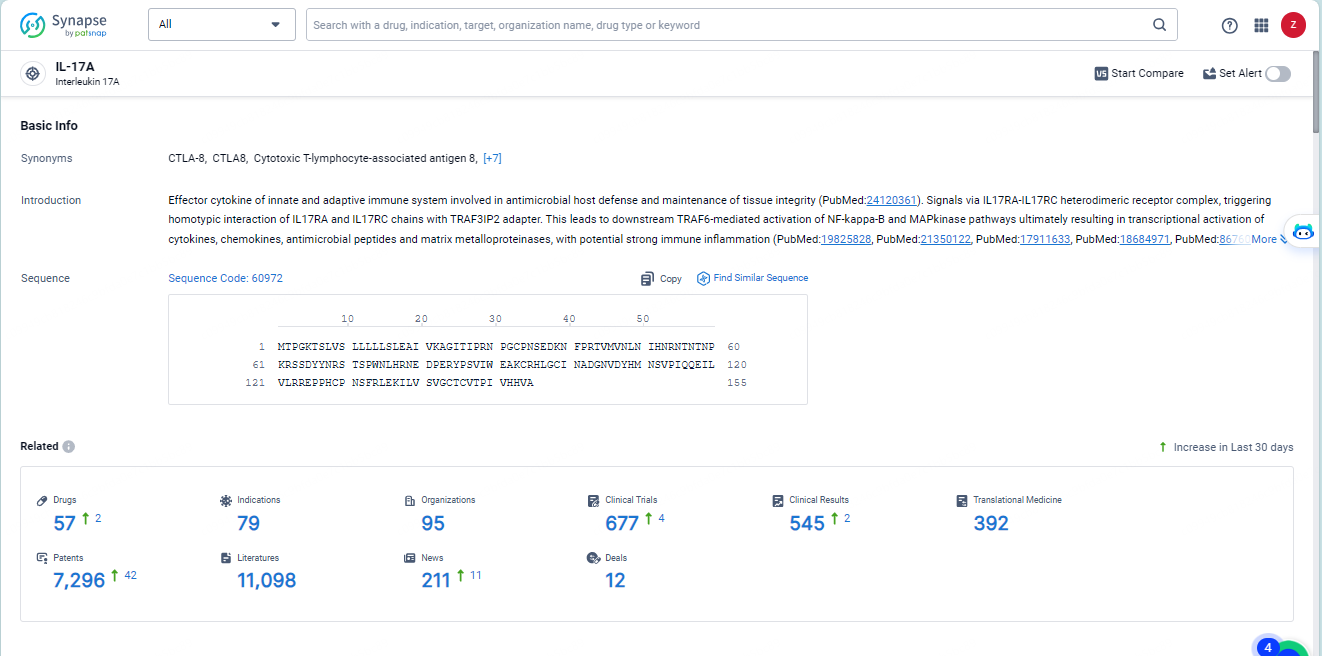
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 28, 2024, there are 57investigational drugs for the IL-17A target, including 79 indications, 95R&D institutions involved, with related clinical trials reaching 677, and as many as 7296 patents.
ZL-1102 indicates that it is a promising drug in the pharmaceutical industry, with a specific focus on addressing immune system diseases and skin conditions. Its progression to Phase 2 on a global scale underscores the potential impact it may have in the field of biomedicine. However, further information about its development in China, as well as additional clinical trial data, would be necessary to fully assess its potential impact and market prospects.