Encoded Therapeutics Gains UK Approval for ETX101 Gene Therapy Trial in Dravet Syndrome
Encoded Therapeutics Inc., a firm specializing in the creation of genetic therapies targeting serious conditions of the brain and nervous system, has disclosed that the UK's Medicines and Healthcare products Regulatory Agency has sanctioned its application for a clinical trial. This approval paves the way for the commencement of the EXPEDITION trial, which will evaluate the efficacy of ETX101 in pediatric patients diagnosed with the SCN1A-positive form of Dravet syndrome.
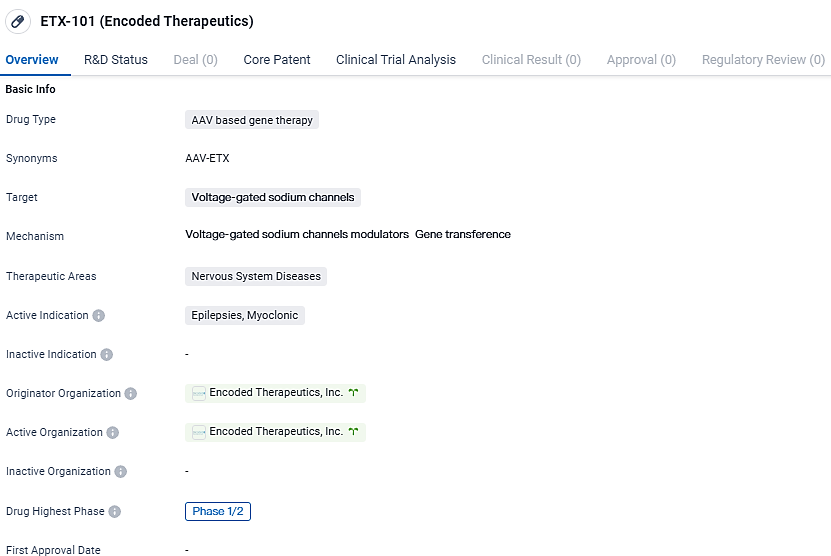
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dravet syndrome stands as the predominant developmental and epileptic encephalopathy, notable for a range of clinical manifestations that include intractable seizures and halted neurodevelopmental progress.
In the UK, the Clinical Trial Authorization (CTA) for the EXPEDITION study is a crucial segment of Encoded's international regulatory approach for the progression of ETX101. This strategy also encompasses the green light for a new investigational drug proposal in the USA, as well as an endorsement under Australia's Clinical Trial Approval system.
"The granting of the UK CTA signifies the third endorsement for ETX101 as we propel the POLARIS global clinical program forward, aiming to bring this innovative single-administration AAV gene regulation treatment to individuals impacted by SCN1A-positive Dravet syndrome across the globe," stated Sal Rico, M.D., Ph.D., and Chief Medical Officer at Encoded.
"ETX101 carries the promise to revolutionize care across various facets of the disease, and our clinical studies are tailored to comprehensively evaluate its effect on young patients. Our team is dedicated to a swift enrollment of participants in association with esteemed researchers, accelerating advancements in treatments for those affected by Dravet syndrome," Sal Rico elaborated.
Professor Andreas Brunklaus, M.D., Ph.D., Lead Investigator of the EXPEDITION trial and Specialist in Paediatric Neurology at Glasgow's Royal Hospital for Children in the UK, remarked, "With the initiation of the EXPEDITION trial, we are addressing the critical demand for development of treatments that can ultimately modify the course of Dravet syndrome for children in the UK. EXPEDITION marks a crucial step in our joint endeavor to uncover therapies that can have a real impact on those suffering from Dravet syndrome."
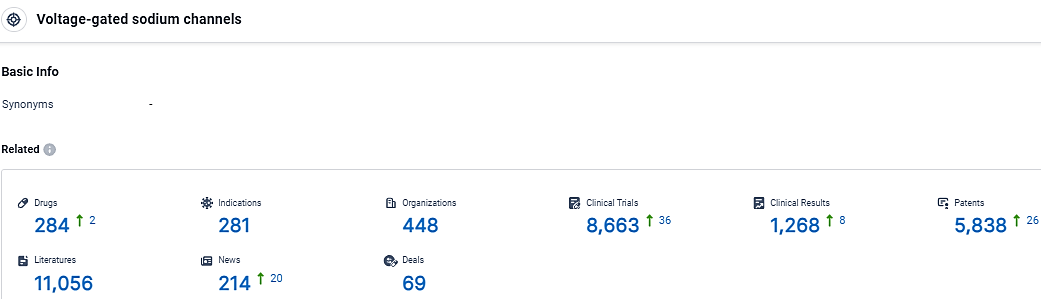
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 29, 2024, there are 284 investigational drugs for the Voltage-gated sodium channels target, including 281 indications,448 R&D institutions involved, with related clinical trials reaching 8663, and as many as 5838 patents.
In ETX101, a transgene encoding an engineered transcription factor under the control of a cell-selective regulatory element is delivered within a clinically-validated capsid to upregulate, or increase, the expression of the endogenous SCN1A gene. This approach is expected to increase production of NaV1.1 protein sodium channels in target neurons in the brain, leading to restored function. By targeting the underlying mechanism, ETX101 has the potential to address the full range of symptoms associated with Dravet syndrome.