Oscotec/Adel Begins Phase 1 Trial of ADEL-Y01 Anti-Tau Antibody for Alzheimer's
Oscotec Inc. and ADEL Inc. have disclosed the commencement of their initial human trial with the first administration of the investigational drug ADEL-Y01 to a healthy volunteer. This trial is part of a research program aiming to assess the potential therapeutic effects of ADEL-Y01 in managing Alzheimer’s disease symptoms. In a collaborative effort, Oscotec and ADEL are working to develop an innovative therapeutic agent that has the potential to alter the progression of the disease by acting on tau protein accumulations observed in Alzheimer's patients' brains. ADEL-Y01 has been specifically engineered to bind to a form of tau protein that is acetylated at the lysine-280 position. This interaction is believed to hinder the development and spread of harmful tau aggregates, which could potentially decelerate the advancement of the disease.
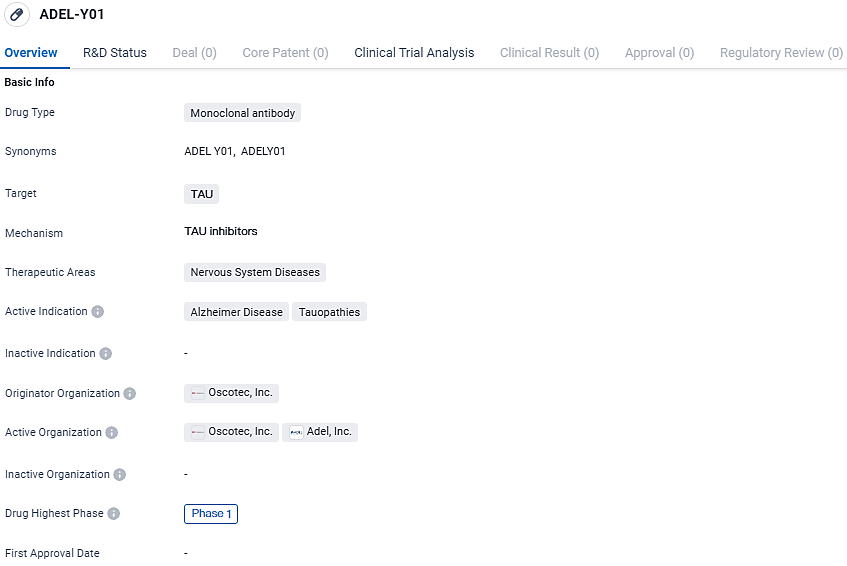
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The initial stage clinical trial, denoted as 1a/1b, being conducted within the United States, is designed to assess the safety profile, capability for patients to endure the treatment, pharmacokinetics, and the therapeutic efficacy of ADEL-Y01 in a cohort of 40 individuals who exhibit no health issues and an additional group of 33 subjects who are experiencing Early Memory Loss associated with Alzheimer's or suffering from the early stages of the Alzheimer's condition.
"Seeing as the protein known as tau exhibits a strong connection with the advancement and the clinical seriousness of Alzheimer's Disease (AD), it is believed that by aiming at tau, it could be possible to decelerate the advancement of the condition. As we deepen our comprehension concerning the critical significance of the tau protein's mid-region, referred to as MTBR, pinpointing the precise epitope becomes vital for the creation of tau-specific medical treatments that yield real clinical advantages," declared SeungYong Yoon, holder of both MD and PhD degrees and the Chief Executive Officer at ADEL.
"Here at Oscotec, we are excited to announce a crucial achievement in spearheading the clinical research in a partnership role with ADEL, and we are determined to persist in the development and offering of groundbreaking treatments that address neurodegenerative diseases," expressed Taeyoung Yoon, the leading executive at Oscotec.
Published in the prior year, the preclinical findings on ADEL-Y01, explored in animal test subjects, showed a notable reduction in memory decline and amelioration of behavioral issues by obstructing the initial transmission and further spread of Tau.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
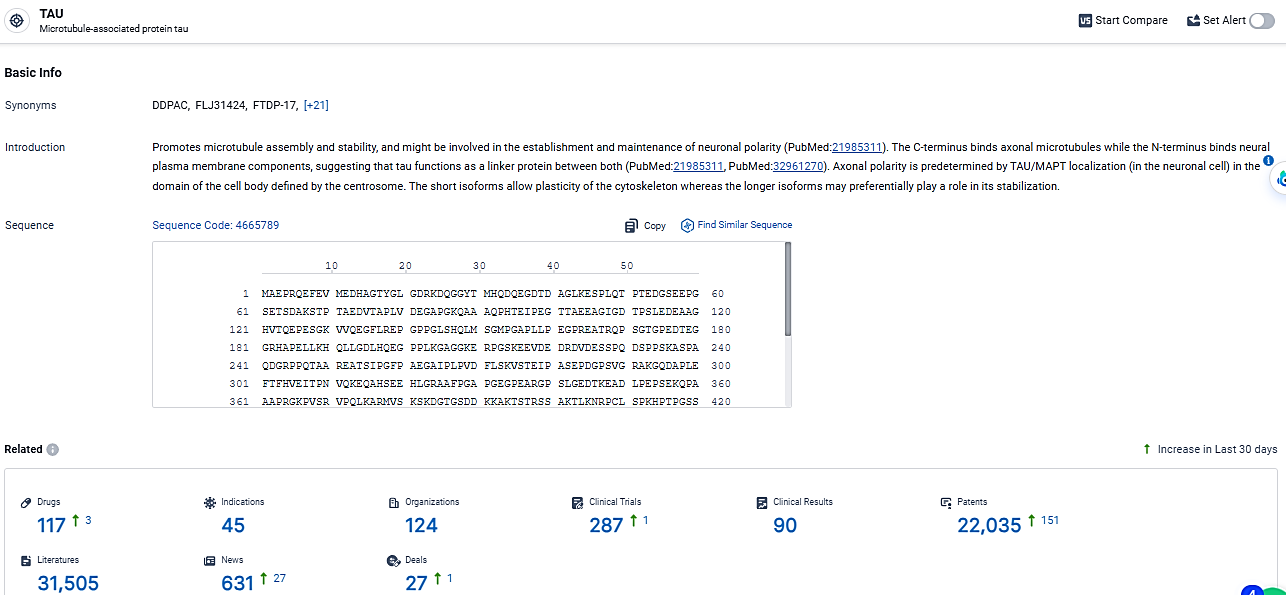 According to the data provided by the Synapse Database, As of February 29, 2024, there are 117 investigational drugs for the TAU target, including 45 indications, 124 R&D institutions involved, with related clinical trials reaching 287, and as many as 22035 patents.
According to the data provided by the Synapse Database, As of February 29, 2024, there are 117 investigational drugs for the TAU target, including 45 indications, 124 R&D institutions involved, with related clinical trials reaching 287, and as many as 22035 patents.
Overall, ADEL-Y01 represents a potential advancement in the field of biomedicine for the treatment of neurodegenerative disorders. However, it is important to note that the information provided is based solely on the given data and does not include any additional subjective interpretations or fictional data. Further research and analysis would be required to fully assess the potential impact and viability of ADEL-Y01 in the pharmaceutical industry.