Encouraging Phase 1 Results for Nxera Pharma and Centessa's New Sleep Deprivation Drug, ORX750
Nxera Pharma Co., Ltd.– previously referred to as Sosei Group or Sosei Heptares-acknowledges the announcement made by Centessa Pharmaceuticals on 10 September 2024, which details encouraging interim clinical results from its Phase 1 trial involving ORX750 in acutely sleep-deprived healthy participants.
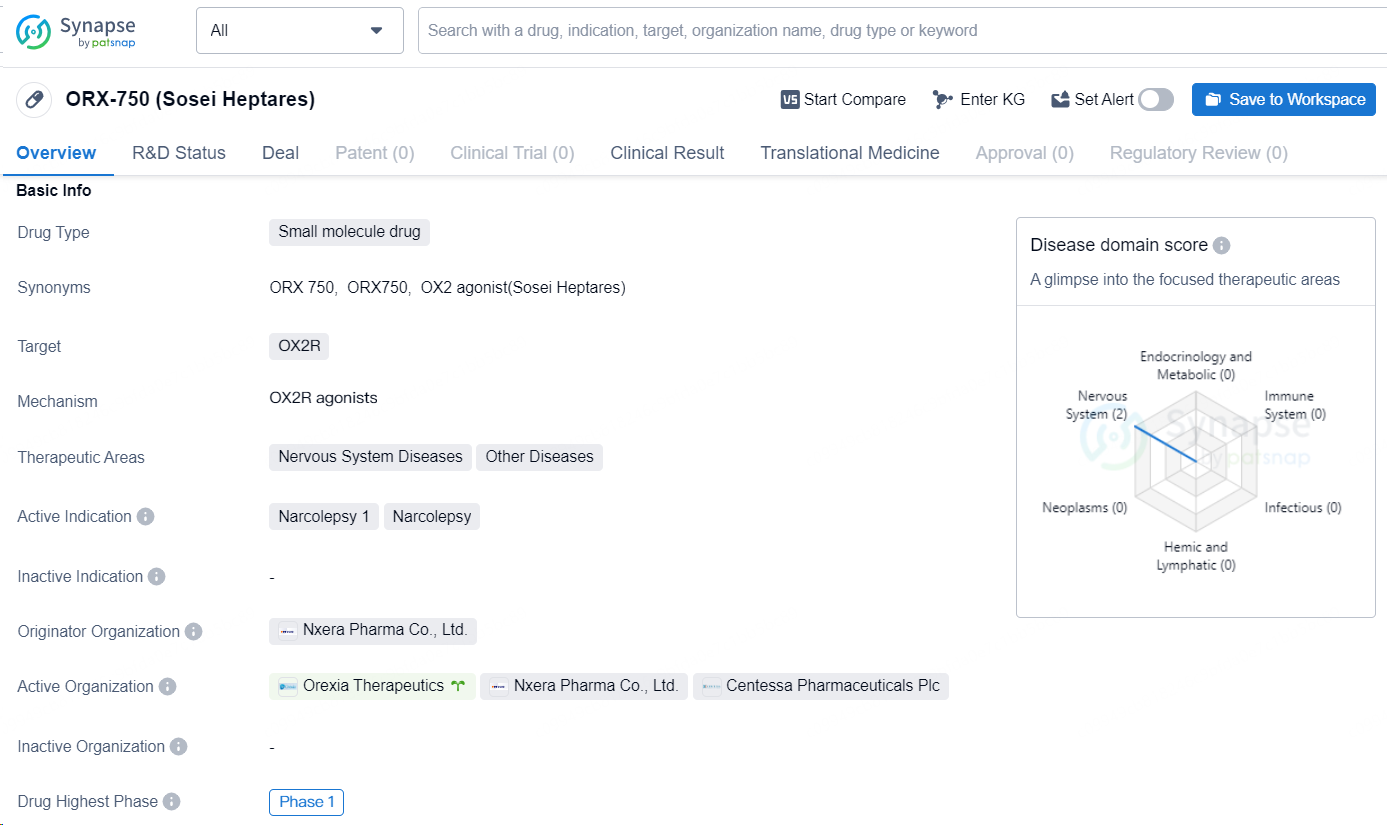
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Centessa reported that ORX750 demonstrated clinically meaningful and statistically significant enhancements in mean sleep latency at the initial two doses examined (1.0 mg and 2.5 mg) during the Maintenance of Wakefulness Test (MWT) when compared to placebo. Specifically, the 2.5 mg dose managed to restore normal wakefulness with a mean sleep latency of 32 minutes as recorded by the MWT.
Furthermore, ORX750 exhibited a positive safety and tolerability profile, with no frequently reported on-target adverse events (AEs) seen with other OX2R agonists, and without any instances of hepatotoxicity or visual disturbances across the three dose levels tested (1.0 mg, 2.0 mg, and 2.5 mg) as per the data cutoff date.
Centessa intends to promptly progress ORX750 into Phase 2 trials for patients with narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH) starting in the fourth quarter of 2024, based on the interim data.
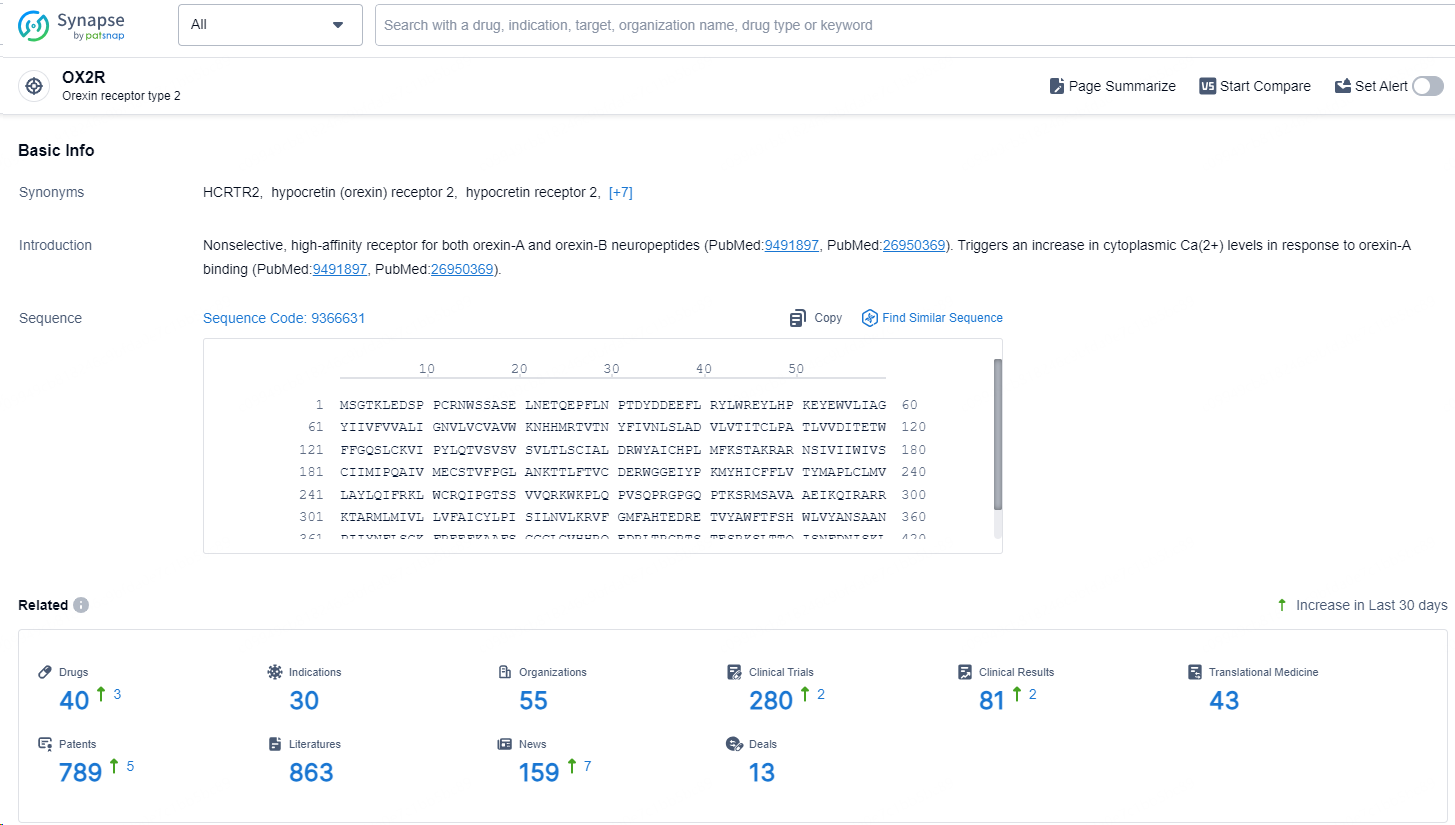
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 14, 2024, there are 40 investigational drugs for the OX2R targets, including 30 indications, 55 R&D institutions involved, with related clinical trials reaching 280, and as many as 789 patents.
ORX-750, developed by Sosei Heptares, is a small molecule drug that targets the OX2R receptor. This drug is intended for the treatment of nervous system diseases as well as other diseases. The active indication for ORX-750 is narcolepsy, with a specific focus on narcolepsy type 1. The originator organization of ORX-750 is Nxera Pharma Co., Ltd. The drug has progressed to the highest global phase of development, reaching Phase 1 clinical trials.