Initial Patient Treated with CX-801 in CytomX’s Phase 1 Trial for Solid Tumors
CytomX Therapeutics, Inc. (Nasdaq: CTMX), a pioneer in the development of masked, conditionally activated biologic treatments, revealed that the initial patient has received a dose of CX-801 as a monotherapy in a Phase 1 clinical trial (NCT06462794) targeting solid tumors. CX-801 is a dually-masked interferon alpha-2b PROBODY® cytokine, which may offer extensive utility in both immune-oncology responsive and non-responsive (cold) tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
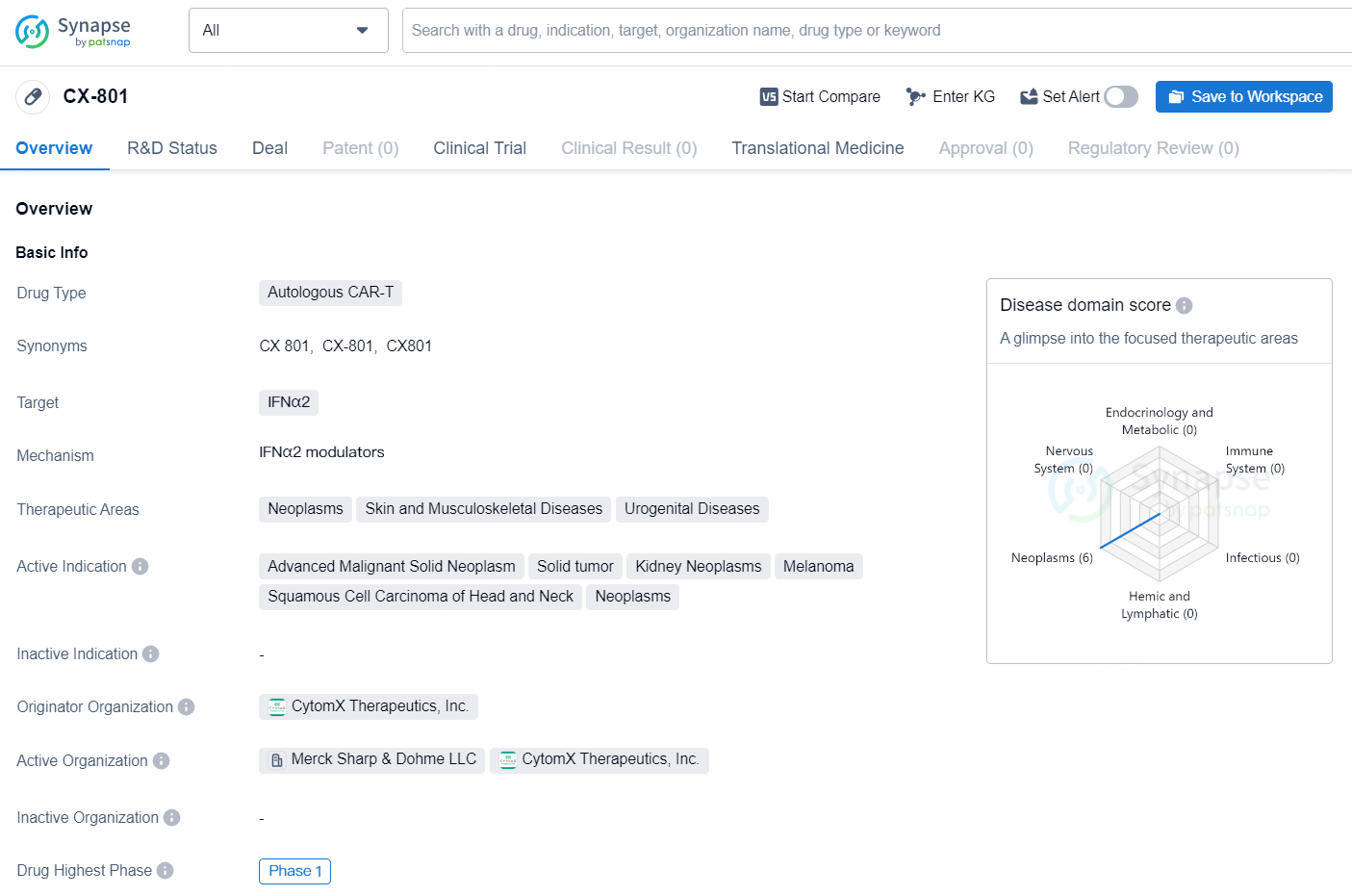 The Phase 1 dose escalation trial of CX-801 aims to assess its safety profile and early indicators of clinical activity as a standalone treatment and in conjunction with Merck’s (referred to as MSD outside the US and Canada) anti-PD-1 therapy, KEYTRUDA® (pembrolizumab). During the dose escalation phase, the study will recruit patients with certain solid tumors, such as advanced melanoma, renal cell carcinoma, and head and neck squamous cell carcinoma. The goal is to determine whether to proceed to Phase 1b with specific dose expansion cohorts based on the indications.
The Phase 1 dose escalation trial of CX-801 aims to assess its safety profile and early indicators of clinical activity as a standalone treatment and in conjunction with Merck’s (referred to as MSD outside the US and Canada) anti-PD-1 therapy, KEYTRUDA® (pembrolizumab). During the dose escalation phase, the study will recruit patients with certain solid tumors, such as advanced melanoma, renal cell carcinoma, and head and neck squamous cell carcinoma. The goal is to determine whether to proceed to Phase 1b with specific dose expansion cohorts based on the indications.
"Interferon-alpha-2b is a robust immune-modulating cytokine that has shown clinical activity in various cancer types, including metastatic melanoma, renal cancer, and bladder cancer. However, its clinical utility has been limited due to significant toxicities when used systemically. CX-801 leverages CytomX’s leading-edge conditional activation platform to retain its potency while broadening the therapeutic index of interferon. This innovation has the potential to make it a critical component of immuno-oncology combination therapies," explained Wayne Chu, M.D., chief medical officer at CytomX Therapeutics.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
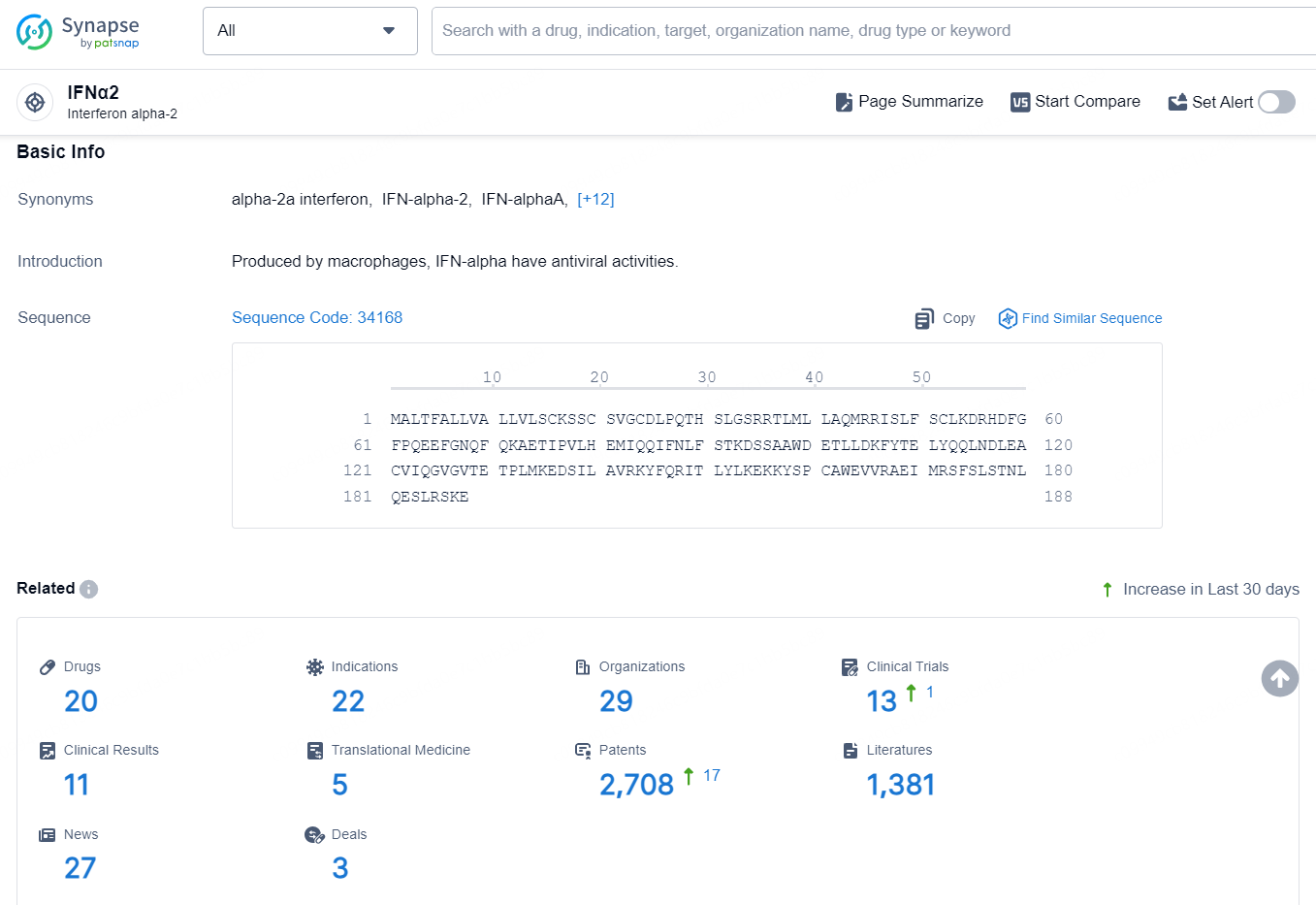 According to the data provided by the Synapse Database, As of September 14, 2024, there are 20 investigational drugs for the IFNα2 target, including 22 indications, 29 R&D institutions involved, with related clinical trials reaching 13, and as many as 2708 patents.
According to the data provided by the Synapse Database, As of September 14, 2024, there are 20 investigational drugs for the IFNα2 target, including 22 indications, 29 R&D institutions involved, with related clinical trials reaching 13, and as many as 2708 patents.
CX-801 is an autologous CAR-T drug that targets IFNα2. It is being developed for the treatment of neoplasms, skin and musculoskeletal diseases, and urogenital diseases. The active indications for CX-801 include advanced malignant solid neoplasm, solid tumor, kidney neoplasms, melanoma, squamous cell carcinoma of head and neck, and other neoplasms.




