EU Approves Merck's KEYTRUDA and Padcev Combo for Advanced Urothelial Cancer
Merck (NYSE: MRK), which operates under the name MSD internationally except in the United States and Canada, has declared that the European Commission (EC) has sanctioned the use of KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, together with Padcev (enfortumab vedotin-ejfv), an antibody-drug conjugate, for the initial treatment of unresectable or metastatic urothelial carcinoma in adults. This authorization comes after the European Society for Medical Oncology and the European Association of Urology endorsed clinical guidelines proposing this combination as the preferred initial treatment for such patients, regardless of platinum-based therapy eligibility.
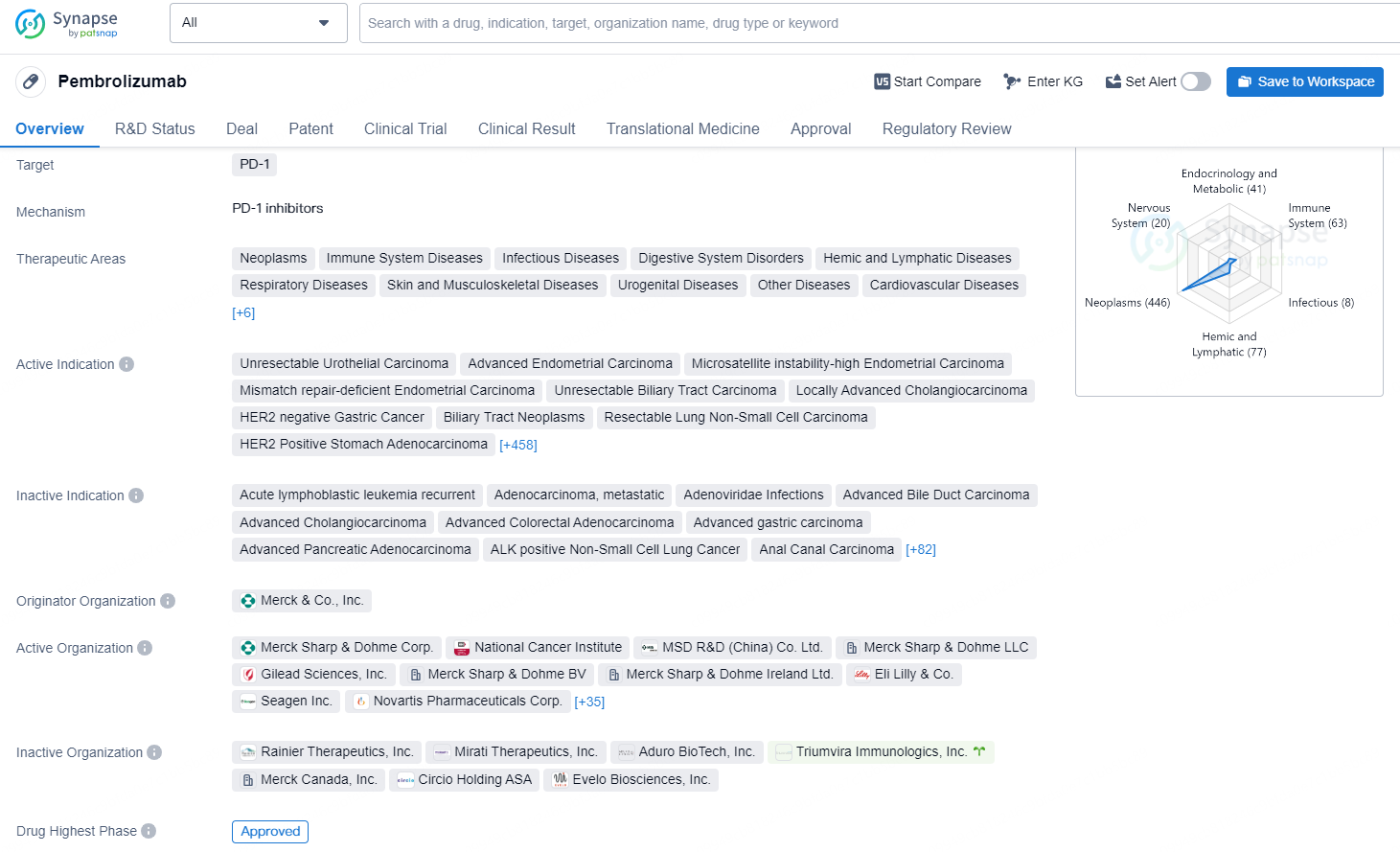
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The European Commission's endorsement also aligns with the favorable recommendation from the Committee for Medicinal Products for Human Use, granted in July 2024, which hinged on findings from the initial interim analysis of the Phase 3 KEYNOTE-A39 trial (also known as EV-302). This trial was conducted in collaboration with Pfizer (formerly Seagen) and Astellas. The KEYNOTE-A39 study showed that the combination of KEYTRUDA and enfortumab vedotin resulted in statistically significant and clinically meaningful enhancements in overall survival (OS) and progression-free survival (PFS) compared to platinum-based chemotherapy (gemcitabine plus cisplatin or carboplatin).
After a median follow-up of 17.3 months (ranging from 0.3 to 37.2 months), the pairing of KEYTRUDA and enfortumab vedotin decreased the risk of death by 53% (HR=0.47 [95% CI, 0.38-0.58]; p<0.00001) compared to platinum-based chemotherapy (with 133/442 [30%] versus 226/444 [51%] events observed, respectively). Additionally, the combination reduced the likelihood of disease progression or death by 55% (HR=0.45 [95% CI, 0.38-0.54]; p<0.00001) compared to platinum-based chemotherapy (with 223/442 [50%] versus 307/444 [69%] events observed, respectively).
“For the first time in many years, adult patients with unresectable or metastatic urothelial carcinoma have a new first-line treatment option that could potentially prolong their lives,” noted Dr. Marjorie Green, senior vice president and head of oncology global clinical development, Merck Research Laboratories. “We are pleased with the European Commission's decision, and we are eager to offer KEYTRUDA as part of this novel therapeutic regimen to patients in the European Union.”
This approval sanctions the marketing of KEYTRUDA combined with enfortumab vedotin for this specific use across all 27 EU member states, along with Iceland, Liechtenstein, Norway, and Northern Ireland. With this approval, KEYTRUDA is now authorized for three indications in bladder cancer and 28 indications overall within the EU. Earlier, KEYTRUDA received EU approval as a monotherapy for treating locally advanced or metastatic urothelial carcinoma in adults who had undergone prior platinum-based chemotherapy, as well as in adults ineligible for cisplatin-containing chemotherapy whose tumors express PD-L1 with a combined positive score (CPS) ≥10, based on findings from KEYNOTE-045 and KEYNOTE-052. In December 2023, KEYTRUDA, in combination with enfortumab vedotin, was also approved in the U.S. for treating adult patients with locally advanced or metastatic urothelial carcinoma.
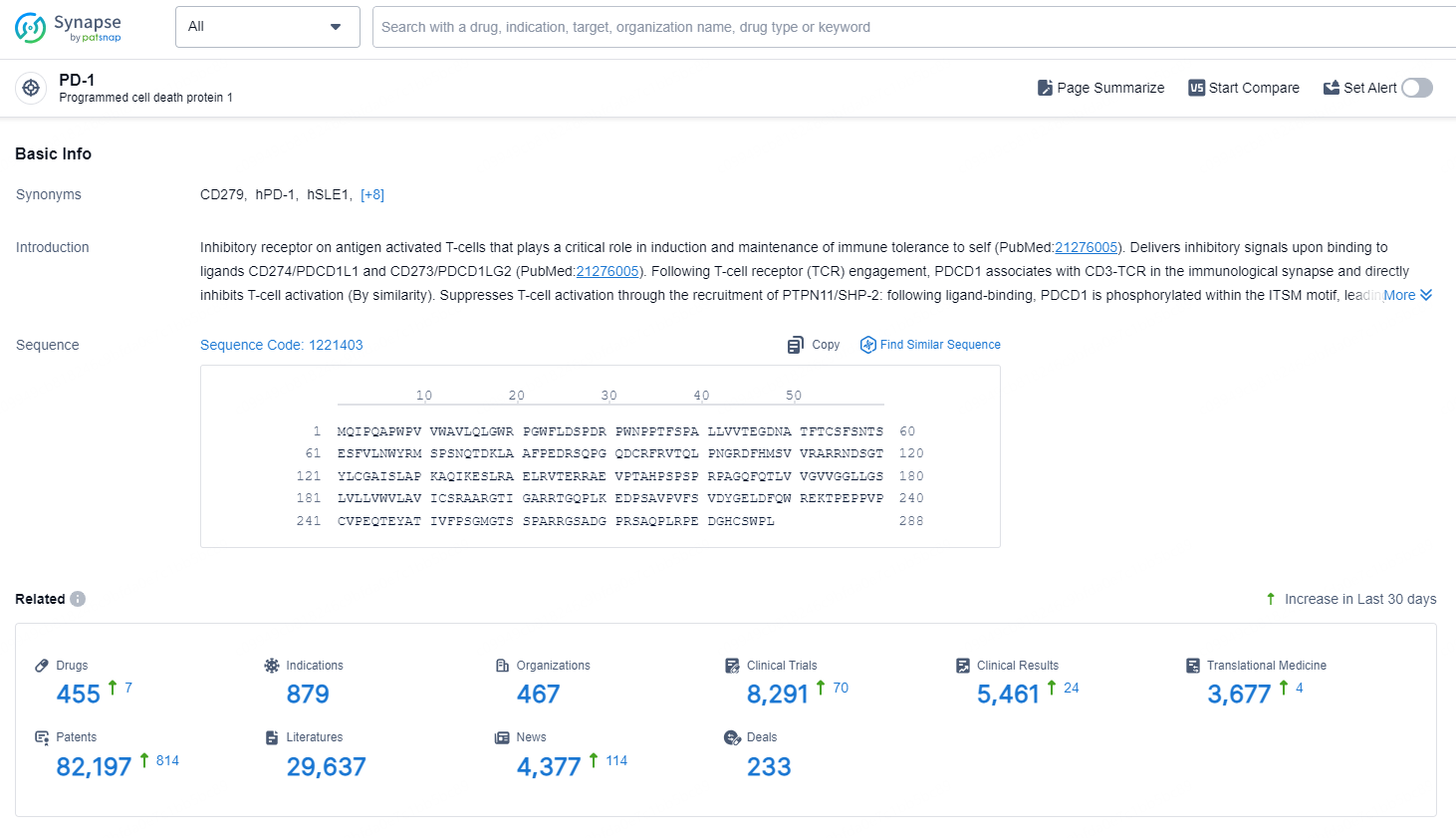
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 4, 2024, there are 455 investigational drugs for the PD-1 targets, including 879 indications, 467 R&D institutions involved, with related clinical trials reaching 8291, and as many as 82197 patents.
Pembrolizumab is a monoclonal antibody that targets PD-1 and is used in the treatment of a wide range of neoplastic and immune system diseases, as well as various other conditions across different therapeutic areas. It is indicated for the treatment of several types of advanced or unresectable cancers such as urothelial carcinoma, endometrial carcinoma, gastric cancer, lung cancer, breast cancer, colorectal cancer, melanoma, and many others. The drug was first approved in the United States in September 2014 and has since received approval in numerous other countries. The originator organization of Pembrolizumab is Merck & Co., Inc.