Antabio Reports Completion of Phase 1 Study for MEM-ANT3310 Treating Serious Hospital Infections
Antabio, a privately-owned biopharmaceutical firm specializing in the creation of innovative and distinct antibacterial therapies targeting critical priority pathogens, especially those causing severe respiratory infections, has reported the successful completion of its Phase 1 clinical trial for MEM-ANT3310 conducted with healthy participants.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Antabio’s MEM-ANT3310 represents an advanced broad-spectrum antibacterial combination specifically designed to combat the escalating issue of antimicrobial resistance in severe hospital-acquired infections. This combination integrates the well-established carbapenem, meropenem (MEM), with ANT3310, a pioneering serine-beta-lactamase (SBL) inhibitor. ANT3310 ensures extensive coverage against high-priority Gram-negative bacteria, including OXA-carbapenem-resistant Acinetobacter baumannii (CRAB), KPC- and OXA-carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa (PA).
The Phase 1 clinical trial was conducted with 72 healthy participants and split into three segments. It assessed the safety, tolerance, and pharmacokinetic (PK) properties of both Single and Multiple Ascending Doses (SAD/MAD) of the intravenous beta-lactamase inhibitor ANT3310 administered alone. Additionally, the study explored potential PK interactions between ANT3310 and meropenem, followed by an evaluation of the combined MEM-ANT3310 during repeated intravenous administration.
ANT3310 was well-tolerated across all dosages with no serious adverse effects, dose-limiting toxicities, or clinically significant abnormalities noted. The PK parameters of ANT3310 aligned with those obtained from preclinical studies in both rodent and non-rodent species, and there was a dose-dependent increase in exposure. The PK profiles of ANT3310 and meropenem were highly compatible with no observed PK interactions.
The positive results from Phase 1 trials support advancing into further patient studies of MEM-ANT3310.
“MEM-ANT3310, with its matching PK profiles and favorable tolerability, holds significant potential for addressing severe infections in hospitalized patients where multidrug resistance is a concern,” said Marc Lemonnier, CEO of Antabio. “With its exceptional broad-spectrum coverage, we anticipate that MEM-ANT3310 will effectively address critical medical needs in high-risk patients, particularly in instances of suspected multidrug-resistant pathogens or polymicrobial infections.”
Currently, Antabio is engaged in a Phase 1 PK study of MEM-ANT3310 in individuals with impaired renal function and is planning a subsequent PK study to analyze the distribution of MEM-ANT3310 in the lung's epithelial lining fluid in healthy volunteers.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
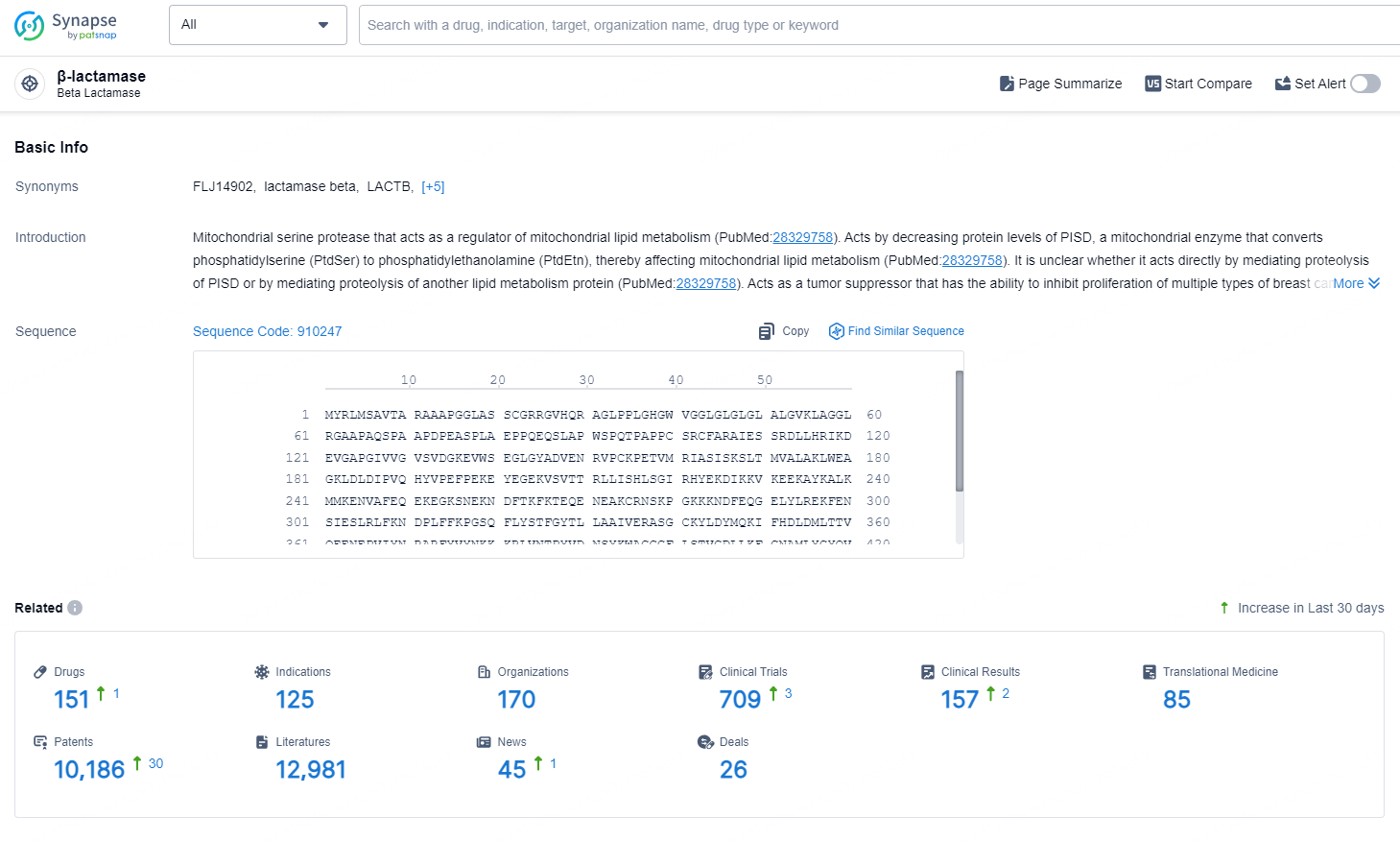
According to the data provided by the Synapse Database, As of September 4, 2024, there are 151 investigational drugs for the β-lactamase targets, including 125 indications, 170 R&D institutions involved, with related clinical trials reaching 709, and as many as 10186 patents.
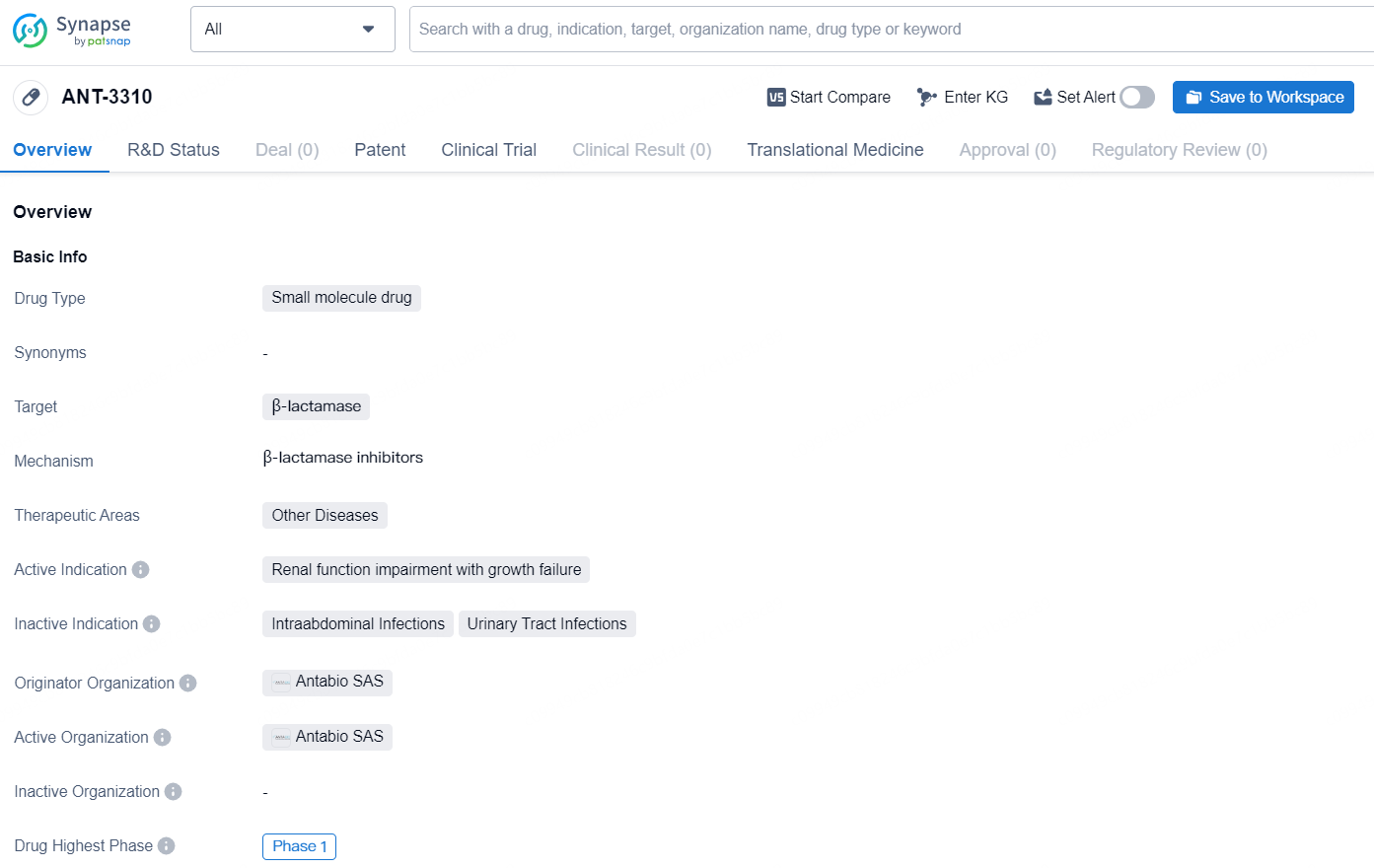
ANT-3310 is a small molecule drug developed by Antabio SAS, and it is designed to target β-lactamase. The drug is currently in its highest global phase, Phase 1, and it is intended for therapeutic areas related to other diseases. The active indication for ANT-3310 is renal function impairment with growth failure. As a small molecule drug, ANT-3310 has the potential to be effective in treating conditions related to β-lactamase and may offer promise in addressing renal function impairment with growth failure. As of now, the drug is still in the early stages of clinical development, but its progress to Phase 1 represents a significant milestone in its potential availability for patients in need.