EU Authority Sanctions Pfizer's Treatment, ELREXFIO®, for Recurring Intractable Multiple Myeloma
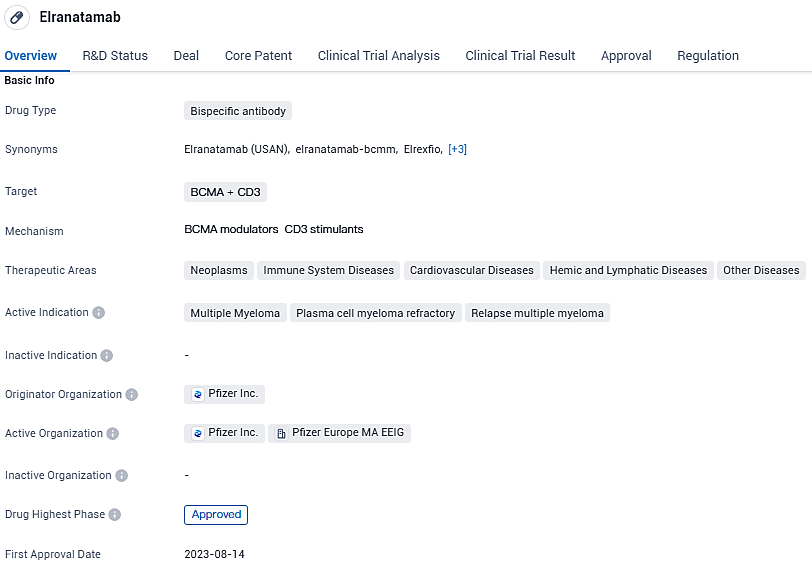
Pfizer Inc. has released an announcement that the European Commission has provided a conditional marketing approval for their product, ELREXFIO (elranatamab). This specific immunotherapeutic agent is approved for usage in adults suffering from multiple myeloma that has not responded to, or has returned after, previous treatments. Candidates for ELREXFIO are those who have undergone a minimum of three previous treatment regimens. These regimens must include therapies with a proteasome inhibitor and an immunomodulatory agent, as well as treatment with an anti-CD38 monoclonal antibody, and their disease must have progressed following the most recent treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ELREXFIO is a readily accessible therapeutic agent, fashioned as a B-cell maturation antigen (BCMA)-CD3-specific bispecific antibody for the immune system, that triggers profound and long-lasting reactions. It is characterized by the feasibility of being well-tolerated and the advantage of straightforward subcutaneous administration.
Annually, over 50,000 individuals within Europe are afflicted with multiple myeloma. Unfortunately, a significant number encounter recurring disease and insensitivity to treatments. Dr. Chris Boshoff, holding the eminent role of Chief Oncology Research and Development Officer as well as Executive Vice President at Pfizer, stated, “The sanctioning of this therapy constitutes a novel and widely obtainable treatment avenue for those grappling with refractory multiple myeloma. Our research regarding ELREXFIO's potential application in preliminary therapy regimes perseveres, aiming to expand its advantages to a larger patient population.”
The interim marketing validation for ELREXFIO has been granted and extends over the entire spectrum of EU territories totaling 27, in addition to Iceland, Liechtenstein, and Norway. This authorization ensues the counsel provided by the European Medicines Agency’s Committee for Medicinal Products for Human Use, which advocated for said provisional marketing authorization on the 12th of October, 2023.
Given the concerns associated with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome, it is imperative for patients to undergo vigilant monitoring of any potential indicative signs and symptoms for a subsequent 48-hour period post-administration of the graduated dosing in the ELREXFIO treatment regiment.
Directions for patients entail staying within accessible vicinity of medical facilities post-dose. However, unlike in the EU, the regulation does not necessitate mandatory hospitalization, nor is there a requirement for patients to remain near medical support following the initial 76-mg dose used for treatment initiation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
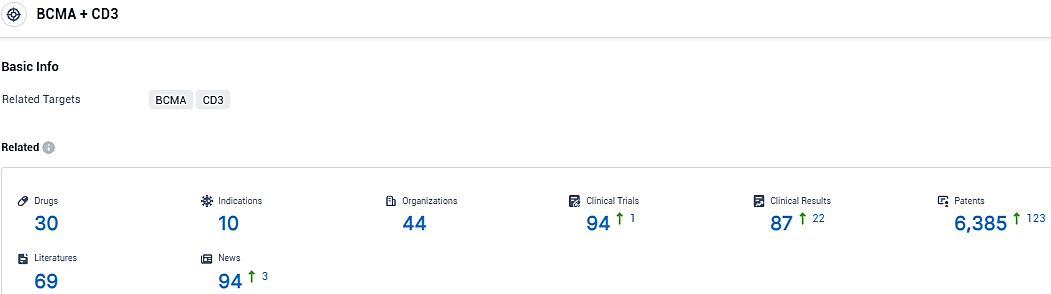
According to the data provided by the Synapse Database, As of December 15, 2023, there are 30 investigational drugs for the BCMA and CD3 target, including 10 indications, 44 R&D institutions involved, with related clinical trials reaching 94, and as many as 6385 patents.
In August 2023, ELREXFIO was approved by FDA under its Accelerated Approval Program, which allows for earlier approval of drugs that treat serious conditions and fill an unmet medical need. ELREXFIO has also received approval in Switzerland and Brazil under Project Orbis, a framework for the concurrent submission and review of oncology drugs among international partners to potentially expedite approvals.