EU Commission Approves BIMZELX® for Hidradenitis Suppurativa, First Biologic Targeting IL-17A and IL-17F
UCB, an international biopharmaceutical firm, has revealed that the European Commission approved the marketing authorization of BIMZELX (bimekizumab). This approval allows its use for treating adults who have active moderate to severe hidradenitis suppurativa and have not adequately responded to standard systemic HS treatments.
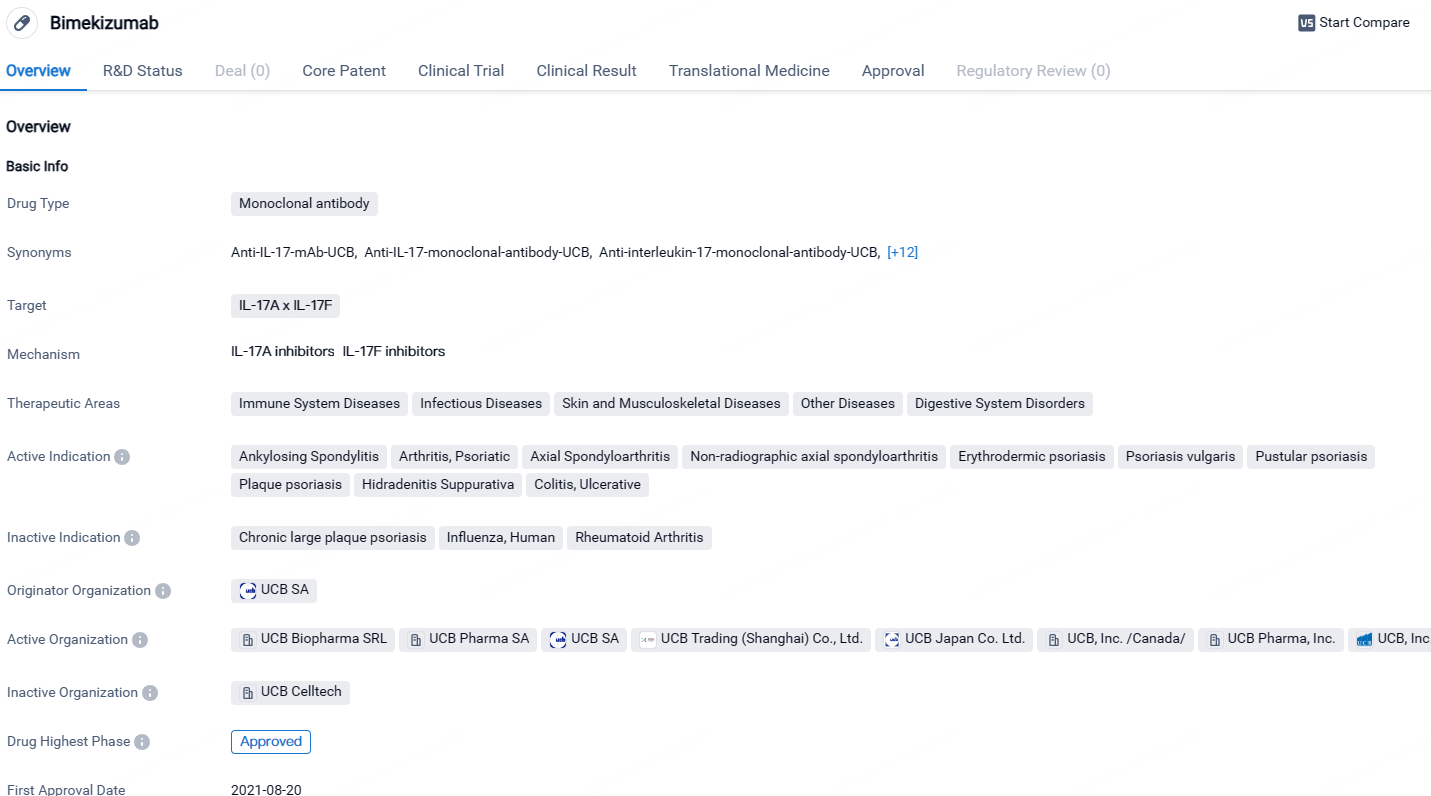
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The European Commission (EC) granted approval following the positive recommendation from the Committee for Medicinal Products for Human Use of the European Medicines Agency in March 2024. This endorsement relied on findings from the Phase 3 trials, BE HEARD I and BE HEARD II, examining the effectiveness and safety of bimekizumab for treating moderate to severe HS.
Professor Dr. Christos C. Zouboulis, who is the head of the Departments of Dermatology, Venereology, Allergology, and Immunology at the Municipal Clinic Dessau, Brandenburg Medical School in Germany, remarked, “The authorization of bimekizumab by the European Commission is a crucial development for the EU community dealing with hidradenitis suppurativa, especially because there are few treatments currently accessible.” He further commented on the community’s efforts, noting, “We are dedicated to bettering the management of hidradenitis suppurativa. Bimekizumab provides a hopeful new treatment path for those suffering from moderate to severe conditions, with Phase 3 data showing significant and lasting benefits through 48 weeks.”
Hidradenitis suppurativa (HS) is a persistent inflammatory skin disease characterized by nodules, abscesses, and fistulas that discharge pus, primarily occurring in the armpits, groin, and buttocks. This condition usually begins in early adulthood and is estimated to affect around one percent of the population across various studies. HS often brings substantial co-morbidities and severely impacts the quality of life for those affected.
Emmanuel Caeymaex, Executive Vice President of Immunology Solutions and Head of U.S., expressed pride in delivering the first and only medicine that specifically targets IL-17A and IL-17F for the hidradenitis suppurativa community. “We are optimistic about the transformative impact of bimekizumab for individuals managing moderate to severe forms of this disease,” he stated.
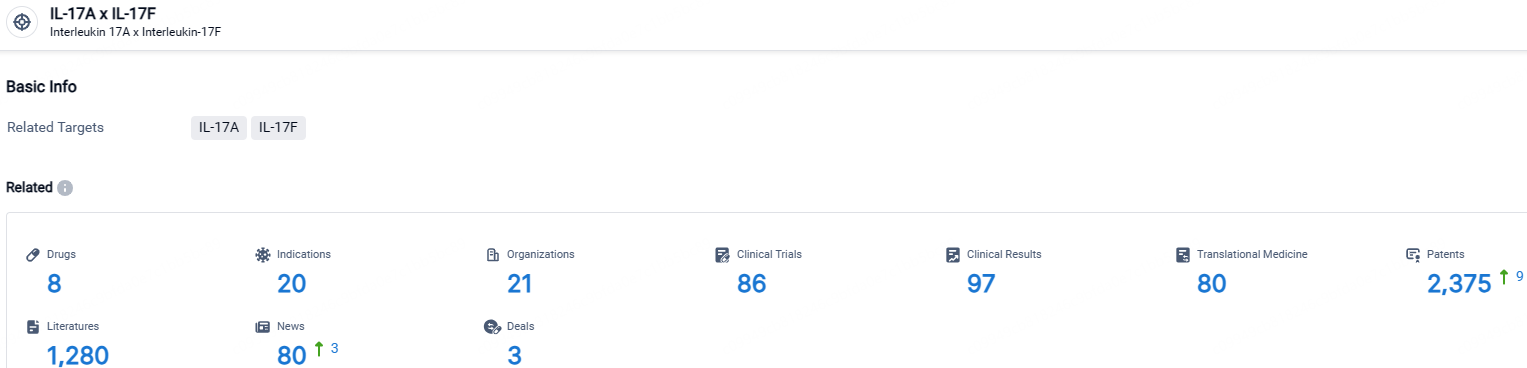
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 23, 2024, there are 8 investigational drugs for the IL-17A and IL-17F target, including 20 indications, 21 R&D institutions involved, with related clinical trials reaching 86, and as many as 2375 patents.
bimekizumab represents a significant advancement in the field of biomedicine, offering a novel treatment option for a wide range of immune system, infectious, skin, and musculoskeletal diseases. Its approval in multiple countries and ongoing regulatory processes in China indicate its potential to address unmet medical needs and improve patient outcomes. Further research and real-world evidence will continue to shed light on the long-term efficacy and safety profile of bimekizumab, solidifying its position in the pharmaceutical industry.