Tavapadon: A New Hope in the Battle Against Parkinson's Disease
Alzheimer's, Parkinson's, and Amyotrophic Lateral Sclerosis (ALS) rank as the three most stubborn tenants among neurodegenerative diseases—in spite of countless therapeutic onslaughts, they remain seemingly impregnable fortresses. Hence, any newly approved medication in this field is frequently touted as a breakthrough of several decades.
The general public's awareness of Parkinson’s disease typically includes symptoms such as uncontrollable shaking, stiffness, and coordination difficulties. Over time, these symptoms worsen, leading to troubles with walking or speaking. Less known, however, are the psychological and behavioral changes Parkinson's patients may experience, such as depression, memory difficulties, fatigue, and sleep disturbances.
The underlying cause of these symptoms is the loss of neurons in the brain that produce dopamine. Levodopa, a precursor to dopamine, is the preferred initial treatment for early Parkinson’s disease, as it helps dopamine-producing neurons generate dopamine. However, long-term use of levodopa can lead to reduced efficacy and cause patients to experience a "wearing-off" phenomenon.
The challenge is finding how to effectively alleviate these symptoms while maintaining sufficient safety. Tavapadon has found a balance in addressing this issue.
In AbbVie's vision, the primary goal of acquiring Cerevel Therapeutics for $8.7 billion was to acquire the antipsychotic drug Emraclidine. According to plan, results from two Phase II studies regarding Emraclidine for the indication of schizophrenia were expected to be announced in the latter half of this year.
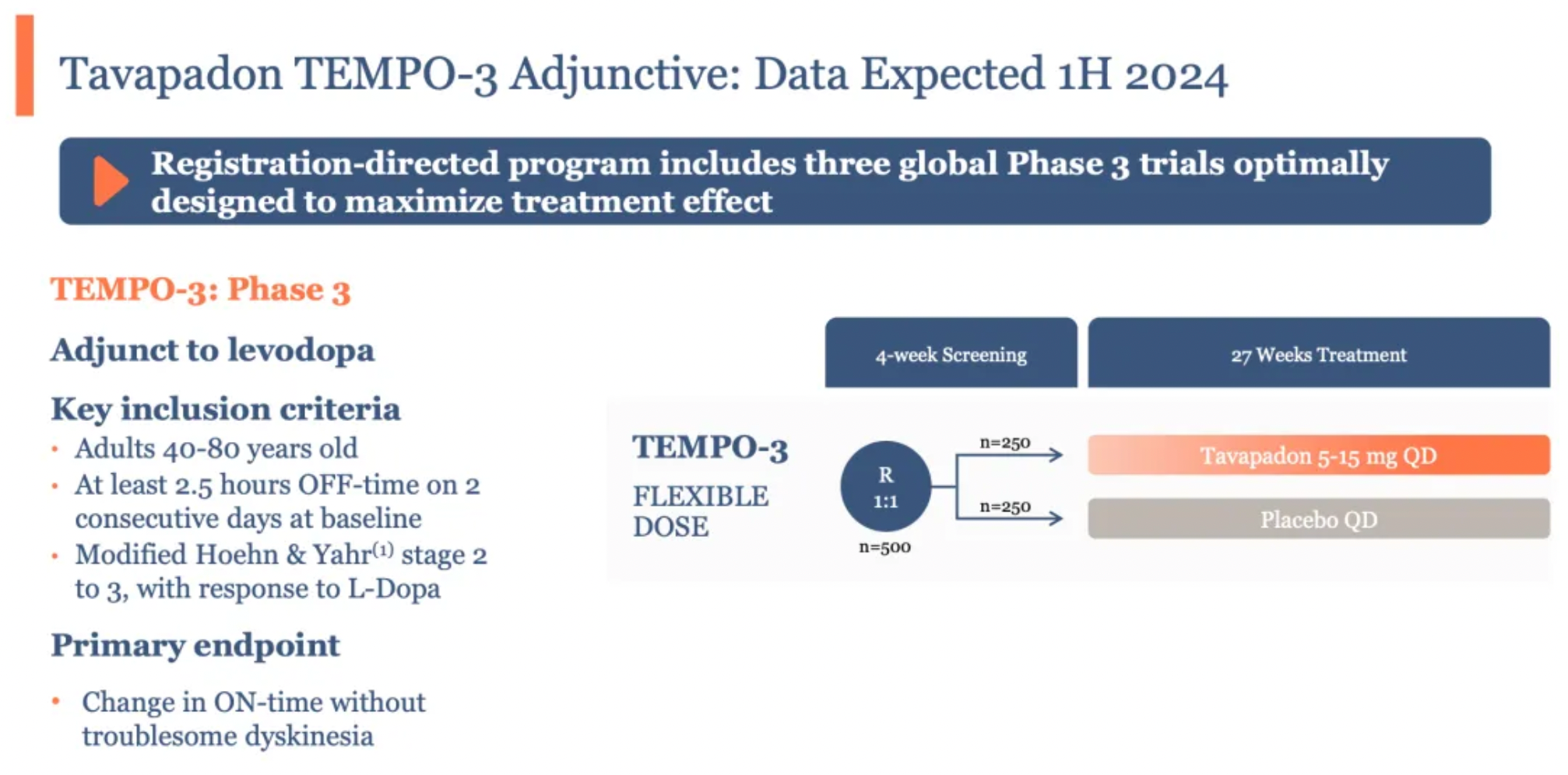
Unexpectedly, another drug, Tavapadon, overshadowed Emraclidine four months later, unexpectedly becoming the first to yield returns for AbbVie. Tavapadon is a once-daily oral partial agonist of the dopamine D1/D5 receptors. On April 18, accompanied by Cerevel’s announcement of successful results from its Phase III study TEMPO-3, Tavapadon’s clinical efficacy received its strongest evidence to date.
The primary objective of the TEMPO-3 study was to assess the effectiveness and safety/tolerability of Tavapadon as an adjunct therapy in Parkinson’s disease patients experiencing motor fluctuations who are already treated with levodopa. Participants were required to have been on a stable dose of levodopa for at least four weeks prior to screening and to maintain the dose and frequency of levodopa usage throughout the trial.
507 enrolled patients were randomized into two groups to receive either Tavapadon or a placebo, administered orally at the same dosage daily for a duration of 27 weeks. The primary clinical endpoint was the change in the total daily "on" hours (hours without troublesome dyskinesia) relative to the baseline.
Long-term administration of levodopa can lead to fluctuations in its efficacy, causing patients with Parkinson's disease to experience sudden improvements or exacerbations in their conditions, similar to being controlled by an on/off switch. This cycling phenomenon greatly affects daily activities, particularly in the "off" state, where patients may experience rigidity and an inability to move.
The TEMPO-3 study results indicated that, compared to a placebo, adjunctive therapy with Tavapadon significantly extended the total daily "on" time (periods without troublesome motor symptoms) by 1.1 hours (1.7 hours vs. 0.6 hours) in patients treated with levodopa, a result that was statistically significant. Additionally, a significant reduction in "off" time was observed in the Tavapadon treatment group for a key secondary endpoint.
In terms of safety, Tavapadon displayed a profile consistent with previous studies, with most adverse reactions being mild or moderate in severity.
It has been reported that the complete results of the TEMPO-3 study will be presented at a forthcoming medical conference and will support the submission of a New Drug Application to regulatory authorities.
The day before the success of Tavapadon was announced, Sage's NMDA receptor allosteric modulator declared a failure in tackling Parkinson's disease. What then makes Tavapadon's mechanism so special, and why was it successful?
Tavapadon is currently the first and only selective partial agonist targeting the dopamine D1/D5 receptors for the treatment of Parkinson's disease. This statement can be divided into two parts; let’s start with the D1/D5 receptor agonists.
The striatum is one of the basal ganglia in the brain, where dopamine acts on two different groups of receptors, primarily D1/D5 and D2/D3. D1/D5 receptors are mainly expressed on the medium spiny neurons (MSNs) in the direct pathway, promoting movement, whereas D2/D3 receptors are expressed on the MSNs in the indirect pathway, acting as inhibitors of movement.
Dopamine binds to the D1/D5 receptors to activate the MSNs of the direct pathway; on the other hand, it binds to the D2/D3 receptors and inhibits the MSNs of the indirect pathway, thus facilitating movement initiation. In the case of Parkinson’s disease, due to the gradual loss of dopamine signaling, there is an imbalance in the activation of the direct and indirect pathways, leading to motor dysfunction. Compounding the problem is the differing affinity of the receptors for dopamine, which in a low dopamine state might preferentially activate the indirect pathway, causing further imbalances.
Current dopaminergic treatments either work like Levodopa, by increasing dopamine levels, or like dopamine agonists, by directly activating dopamine receptors in the striatum. Most dopamine agonists currently target the D2/D3 receptors, leading to selective activation of neurons in the indirect pathway, but the motor effects produced by inhibiting the indirect pathway are somewhat inferior to those of Levodopa. In addition to increasing dopamine levels, Levodopa also activates all subtypes of dopamine receptors, effectively giving a dual boost. On the balance scales of dopaminergic therapy, besides effectiveness, there are safety and tolerability, both of which might also be affected by the selectivity of dopamine receptor subtypes.
Dopamine agonists not only activate receptors within the striatum, but also stimulate receptors in extrastriatal brain regions. It is important to note that D2/D3 receptors are expressed in areas outside the striatum, suggesting that D2/D3 receptors might lead to effects beyond motor activation, such as hallucinations and other cognitive issues.
Given the above, it might be worthwhile to explore D1/D5 receptor agonists instead of those selectively binding to D2/D3 receptors.
Why consider partial agonists? There have been several challenges in the development of selective D1/D5 dopamine agonists, including cardiovascular and motor disorder adverse events, as well as issues with tolerance and bioavailability.
However, some of these adverse effects may be associated with full activation of D1/D5 receptors. By using D1/D5 receptor partial agonists, which have a less intense activating effect, many of these adverse outcomes can be substantially avoided.
The success of the TEMPO-3 study corroborates the design approach of Tavapadon. By selectively activating D1/D5 dopamine receptors along the nigrostriatal pathway, Tavapadon helps improve motor control and avoids the side effects caused by overstimulation of D2/D3 receptors. Furthermore, as a partial agonist with a 24-hour half-life, Tavapadon also prevents motor disorders that can arise from excessive stimulation of dopamine receptors.
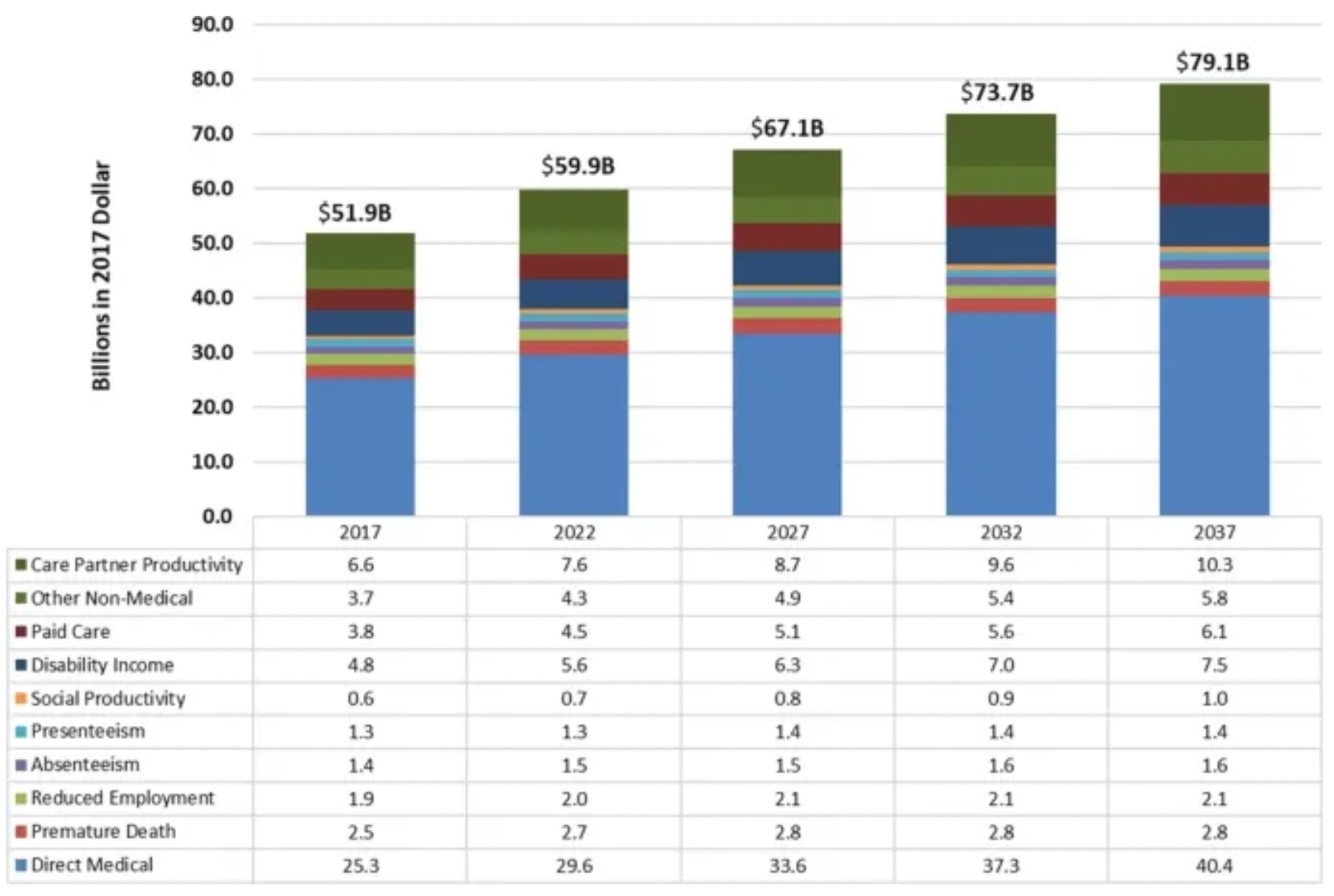
Parkinson's disease is currently the fastest-growing neurodegenerative disease. As of 2022, it is estimated that nearly one million people in the United States are affected by Parkinson's disease, and this number is expected to increase to more than 1.6 million by 2037, as the population ages.
At the same time, Parkinson's disease imposes a significant economic burden on society. It was estimated that the societal burden of Parkinson's disease in the U.S. was $51.7 billion in 2017, which increased to $54.2 billion just a year later, with projections reaching $79.1 billion by 2037.
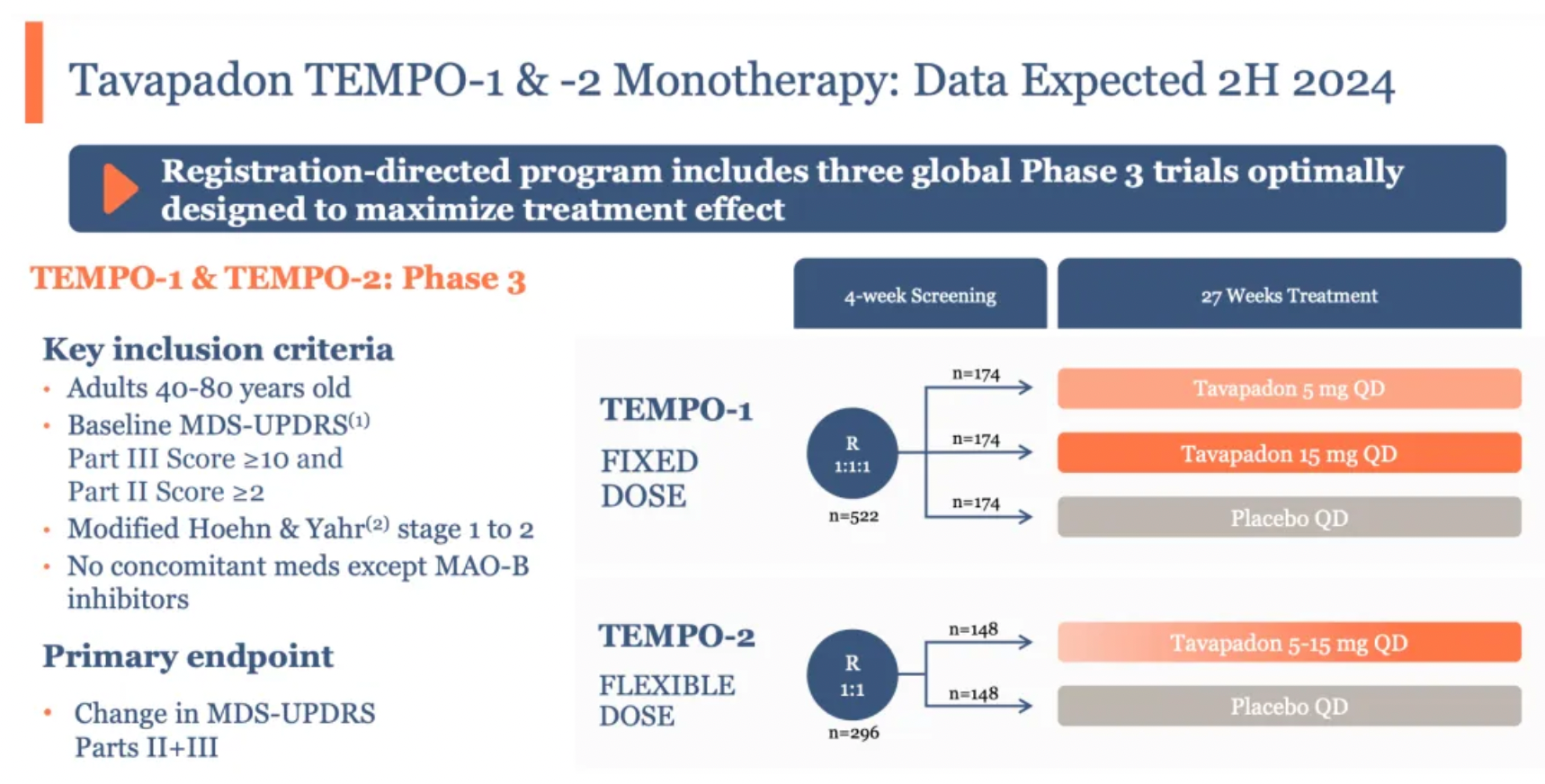
For Tavapadon, the results of two Phase III studies (TEMPO-1 and TEMPO-2), which will evaluate its efficacy as a monotherapy for treating Parkinson's disease, are set to be revealed in the latter half of the year. Furthermore, the open-label extension study, TEMPO-4, will assess the long-term safety and tolerability of Tavapadon.
What opportunities will this series of studies bring for Tavapadon? Analysts' views are mixed.
Some analysts believe that Tavapadon might only be "a small part of revenue contribution," with an estimated peak annual sales revenue of $532 million in the U.S. However, others see the potential for Tavapadon to be a blockbuster, because its clinical efficacy appears to be better than other recently approved Parkinson's drugs.
Regardless, this is good news for AbbVie. Previously, AbbVie collaborated with other companies in the area of advanced Parkinson's but was absent in the early stages. Tavapadon fills this gap.




