European Commission Approves Biogen’s QALSODY® for Rare Genetic ALS
Biogen Inc. revealed that the European Commission has provided marketing approval under extraordinary conditions while preserving orphan status for QALSODY (tofersen) for the therapy of adult patients suffering from amyotrophic lateral sclerosis linked to a mutation in the superoxide dismutase 1 gene (SOD1-ALS). QALSODY stands as the pioneering treatment sanctioned in the European Union that addresses a genetic origin of ALS, which is also referred to as motor neuron disease.
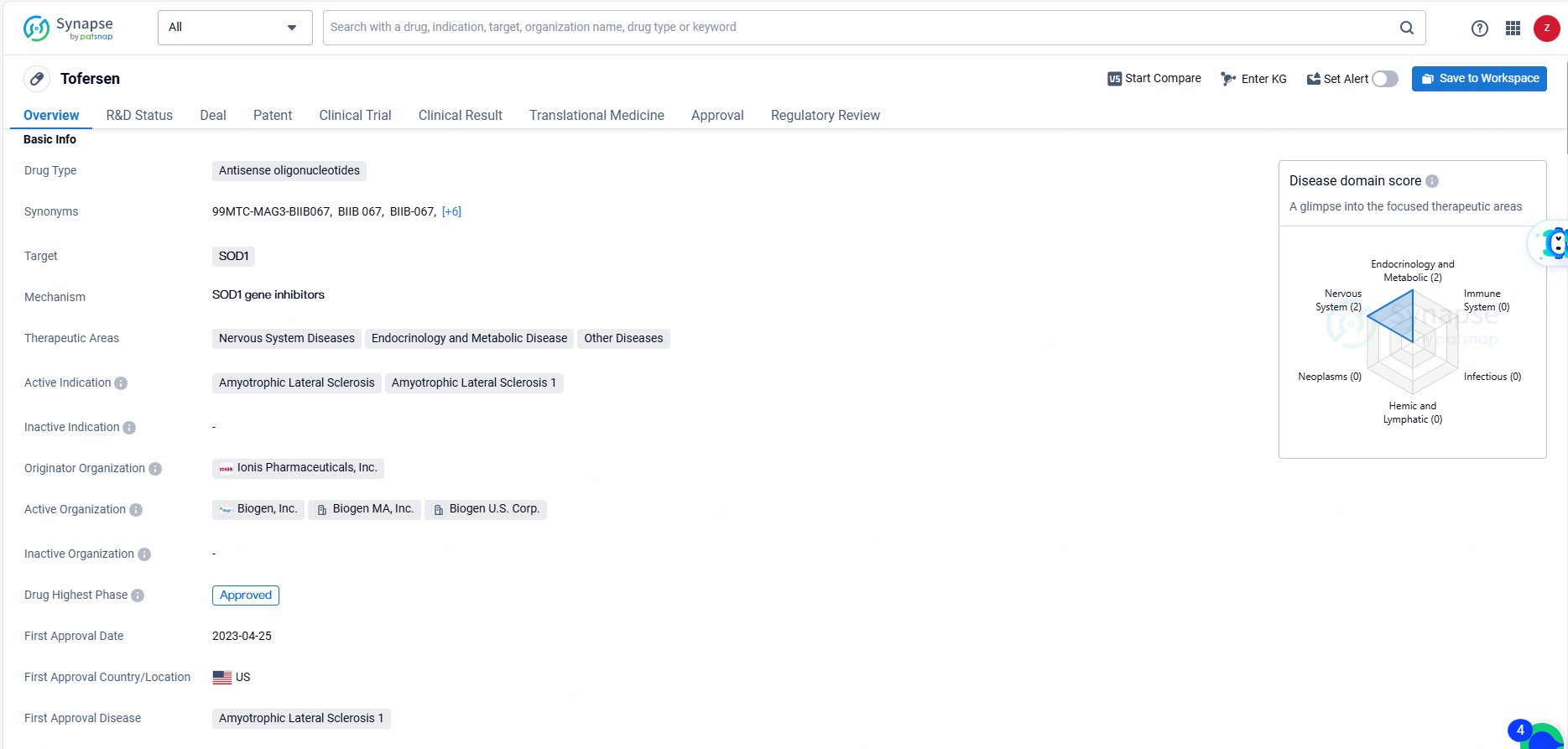
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The endorsement of QALSODY by the European Commission highlights the relentless commitment of the ALS community – including individuals with ALS, their families, researchers, healthcare professionals, and activists – who have collaborated over the last twenty years to develop this crucial new therapy for those with SOD1-ALS," expressed Stephanie Fradette, Pharm.D., Head of the Neuromuscular Development Unit at Biogen.
"We are partnering with the medical field and local officials to make QALSODY available to individuals with SOD1-ALS throughout the region as swiftly as possible," added Fradette.
QALSODY has received marketing authorization under exceptional conditions, a status recommended when the benefit/risk evaluation of a therapy is deemed positive but, due to the rarity of the condition, comprehensive data collection is unfeasible under standard usage situations. The European Medicines Agency has recommended maintaining QALSODY's designation as an orphan medicinal product.
"The approval of QALSODY marks a significant change in SOD1-ALS treatment, bringing hope to patients and their families who have long anticipated a significant advancement," stated Philip Van Damme, M.D., Ph.D., Professor of Neurology and Director of the Neuromuscular Reference Center at the University Hospital Leuven in Belgium. "The European Academy of Neurology has endorsed new ALS treatment guidelines, advocating for QALSODY as the first-line treatment for individuals with SOD1-ALS."
Biogen is dedicated to collaborating with all stakeholders to ensure that this treatment is accessible to qualifying patients in Europe. Through its early access program, Biogen has provided QALSODY to approximately 330 individuals with SOD1-ALS across 18 EU nations. The United States has also approved QALSODY, and Biogen is in discussions with regulatory bodies in other regions.
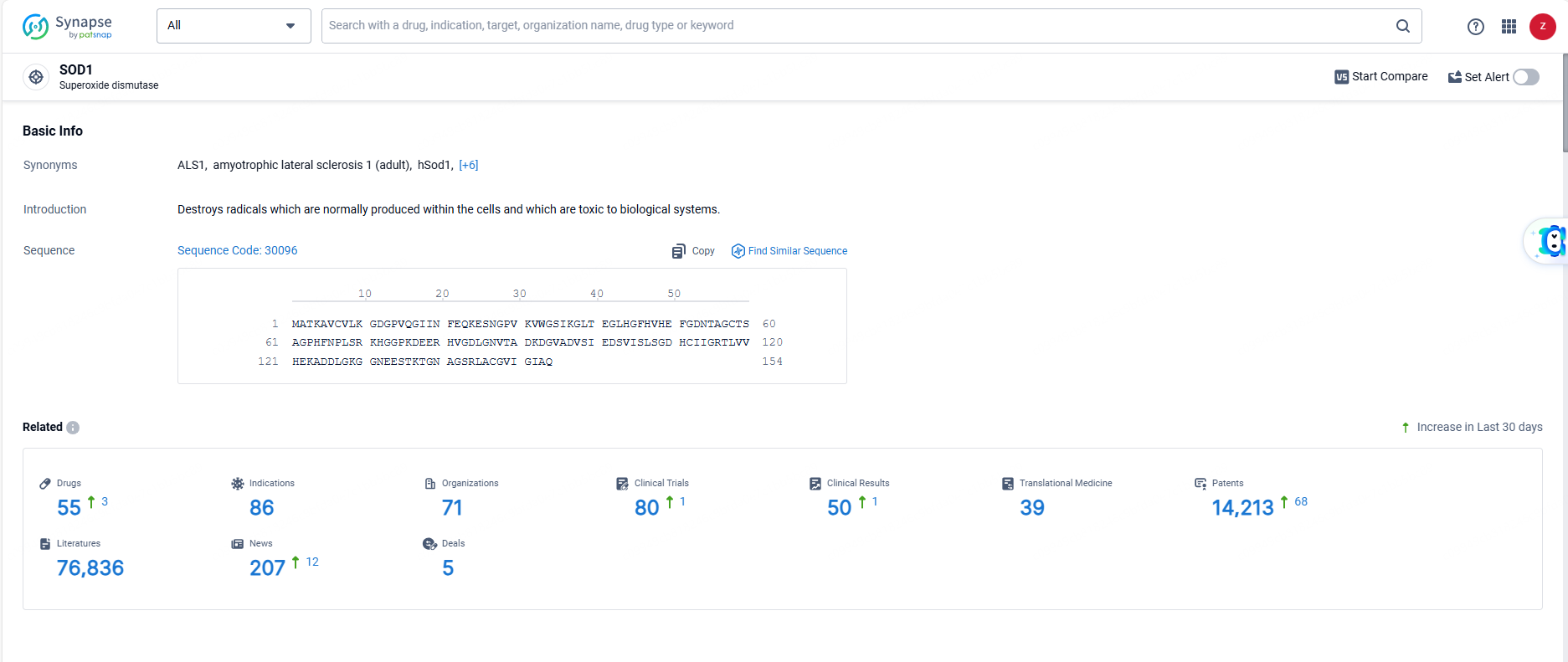
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 7, 2024, there are 55 investigational drugs for the SOD1 target, including 86 indications, 71 R&D institutions involved, with related clinical trials reaching 80, and as many as 14213 patents.
Tofersen's approval as an antisense oligonucleotide drug targeting SOD1 represents a significant advancement in the field of biomedicine, particularly for the treatment of ALS. Its regulatory status, first approval date, and therapeutic potential position Tofersen as a notable addition to the pharmaceutical armamentarium for addressing neurodegenerative diseases.