Evommune Begins Phase 2 Study of Oral MRGPRX2 Antagonist EVO756 in Chronic Inducible Urticaria
Evommune, Inc., a clinical-stage biotechnology firm focused on creating novel treatments for immune-mediated inflammatory conditions, has announced the initiation of a Phase 2 trial for EVO756, with the first patient now enrolled. The trial targets adults with CIndU. EVO756 is a highly selective, potent small molecule antagonist of the mas-related G-protein coupled receptor X2 (MRGPRX2). This innovative therapy offers a new, targeted treatment approach that could allow for once-daily oral dosing, potentially eliminating the severe side effects associated with existing treatments.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
As we advance with our clinical development strategy for EVO756, we are launching this Phase 2 study at 15 locations across the U.S. "Patients with CIndU have few treatment choices, and we think that a new, highly potent, selective agent, administered orally once a day, could offer significant therapeutic advantages," stated J. Mark Jackson, MD, Vice President of Clinical Development at Evommune.
This multi-center study aims to assess the safety and effectiveness of EVO756 in approximately 30 individuals suffering from either symptomatic dermographism or cold urticaria, the two most prevalent types of CIndU. The primary efficacy measures will include changes from baseline in disease-specific provocation thresholds, serving as objective indicators to measure disease severity and response to treatment. EVO756 will be taken orally once per day over a four-week period. Safety and efficacy will be monitored at weekly intervals during the treatment, with patients acting as their own control group.
"The commencement of this trial signifies another key milestone in our commitment to delivering the therapeutic value of MRGPRX2 antagonism to a broad array of mast cell-related conditions," commented Daniel J. Burge, MD, Senior Vice President of Clinical Development at Evommune. "Building on the success of our earlier proof-of-concept study with EVO756 earlier this year, we are also scheduled to begin a Phase 2b trial for chronic spontaneous urticaria in the second quarter of 2025."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
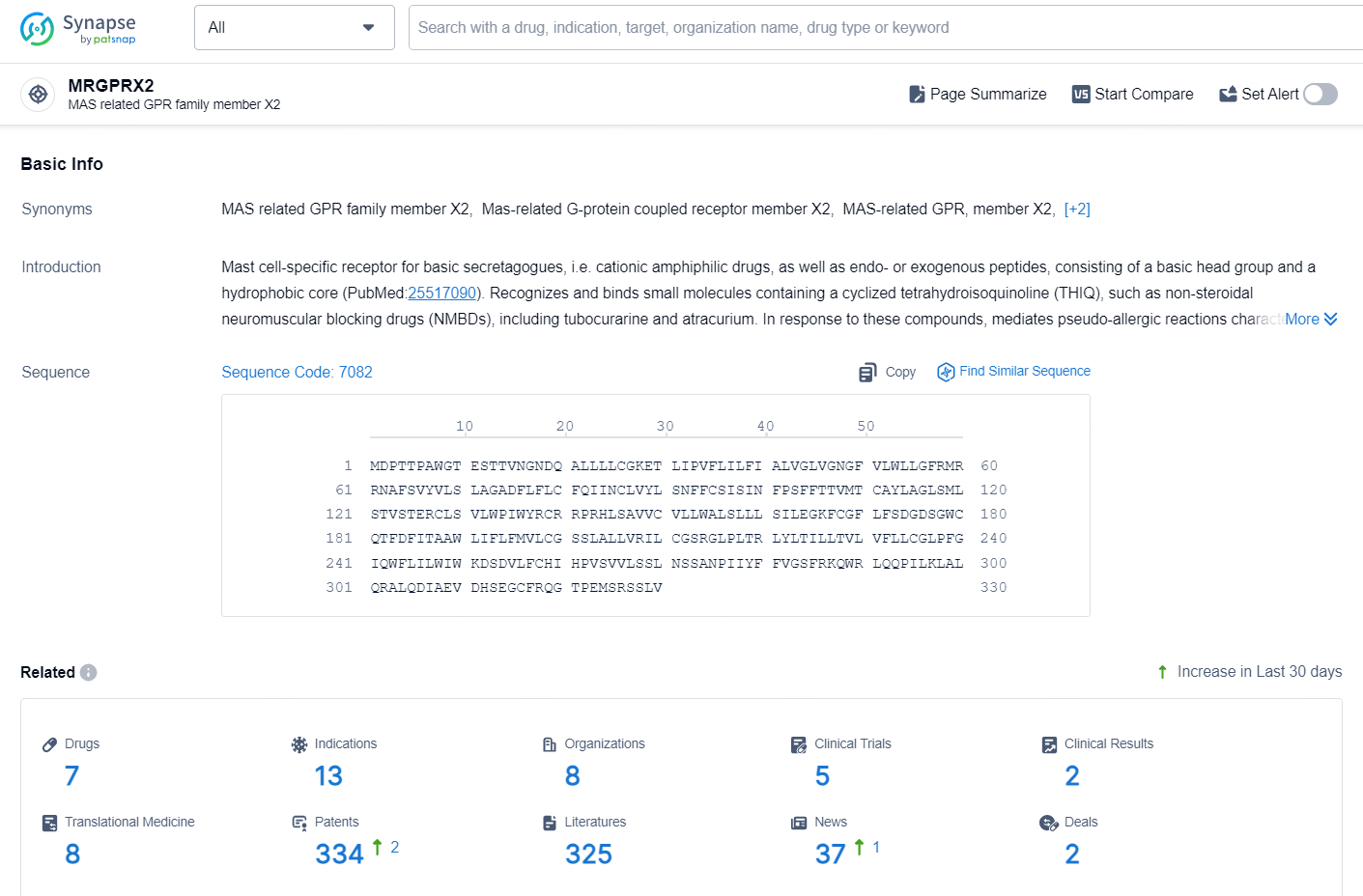
According to the data provided by the Synapse Database, As of September 4, 2024, there are 7 investigational drugs for the MRGPRX2 targets, including 13 indications, 8 R&D institutions involved, with related clinical trials reaching 5, and as many as 334 patents.
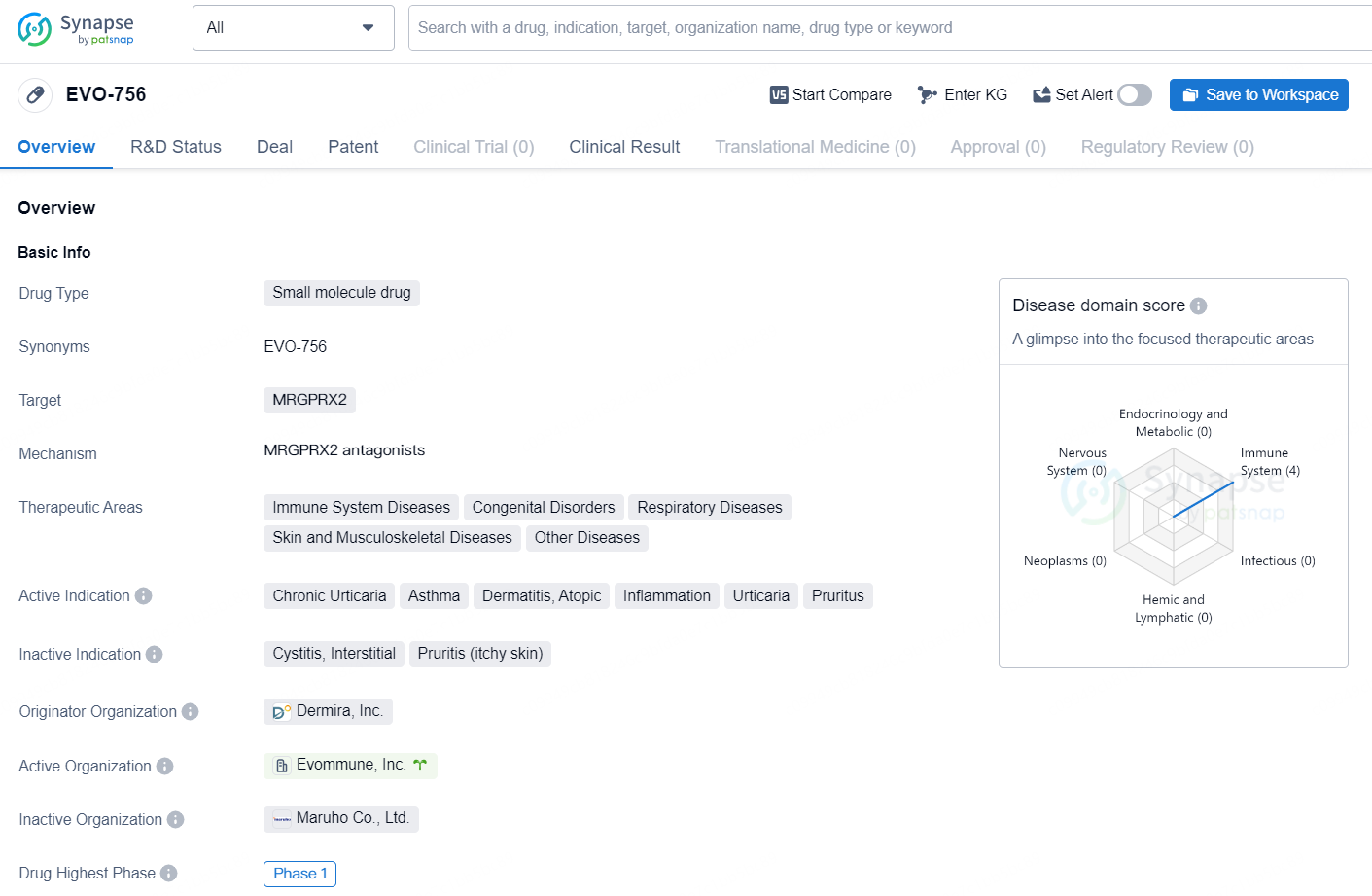
EVO-756 is a small molecule drug developed by Dermira, Inc. The drug targets the receptor MRGPRX2 and has been developed for the treatment of various therapeutic areas, including immune system diseases, congenital disorders, respiratory diseases, and skin and musculoskeletal diseases. Additionally, it is being investigated for its potential in addressing other diseases. The active indications for EVO-756 include chronic urticaria, asthma, dermatitis (atopic and other types), inflammation, urticaria, and pruritus.