ExeGi Pharma Reveals Initiation of Participant Inclusion for EXE-346 in the PROF Study
ExeGi Pharma, an innovative leader in the biopharmaceutical industry focusing on the creation and market introduction of groundbreaking live biotherapeutic products, recently declared the initiation of participant enrollment for its significant PROF Trial. This clinical study aims to examine the efficacy of EXE-346, an emergent live biotherapeutic product, in managing increased bowel movement frequencies in individuals who have undergone an ileal pouch-anal anastomosis procedure.
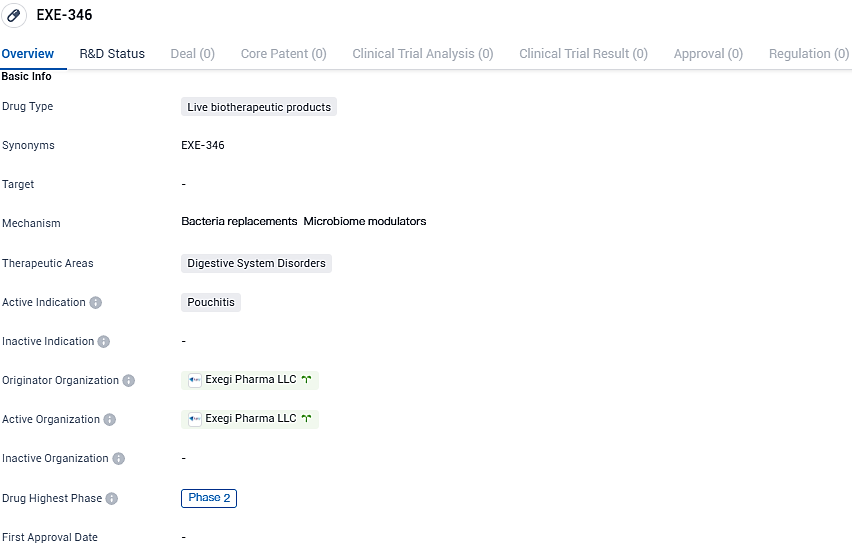
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The procedure known as ileal pouch-anal anastomosis is frequently performed on individuals suffering from ulcerative colitis and results in significant changes to the structure of the digestive system. Some patients who undergo this operation find themselves struggling with persistent, excessive bowel movements, which dramatically affects their daily living. EXE-346 is being developed as a hopeful remedy to mitigate this concern.
Dr. Hans Herfarth, who is leading the PROF Trial and is also the head of the Multidisciplinary IBD Center at the University of North Carolina, stated, "We are filled with optimism regarding the prospects of EXE-346. The trial marks an important advancement in our pursuit to enhance life for those dealing with the aftermath of ileal pouch-anal anastomosis."
Live biotherapeutics, not unlike probiotics, comprise living microorganisms beneficial to health, but unlike probiotics, they must obtain FDA sanctioning to be marketed as medication. The data garnered from the PROF Trial is expected to shed light on how effectively EXE-346 can be used in treating IPAA patients who are burdened with uncontrollable and frequent bowel movements.
Marc Tewey, the CEO of ExeGi Pharma, expressed his enthusiasm about commencing this ground-breaking research, highlighting that enrolling the initial patient is a significant event. "We are completely dedicated to revolutionizing treatment options and believe that EXE-346 might signal the dawn of a transformative period in the management of conditions related to the pouch disorders."
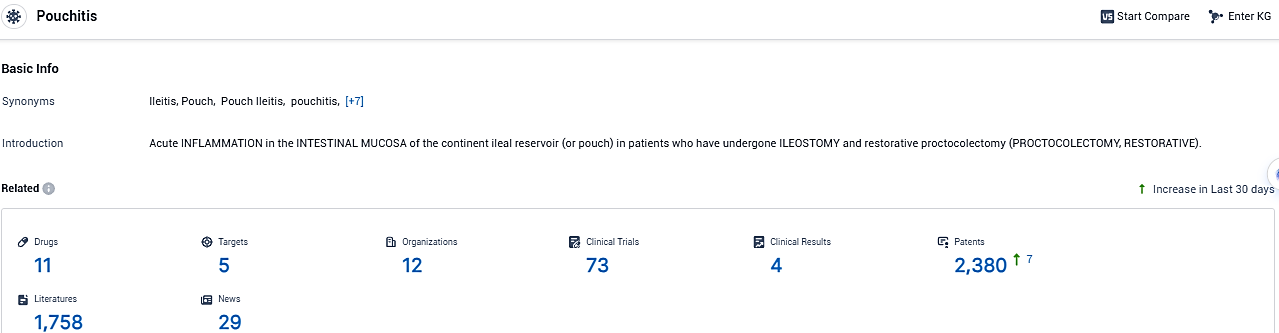
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of December 15, 2023, there are 11 investigational drugs for the Pouchitis, including 5 targets, 12 R&D institutions involved, with related clinical trials reaching 73, and as many as 2380 patents.
EXE-346 has reached Phase 2 of clinical development globally, indicating its potential as a therapeutic option. However, additional research and regulatory processes are necessary to determine its safety and efficacy.