Exploring Dihydroergotamine Mesylate's Revolutionary R&D Successes
Dihydroergotamine Mesylate's R&D Progress
Dihydroergotamine Mesylate is a small molecule drug that primarily targets the 5-HT1D receptor. It falls under the therapeutic area of Nervous System Diseases and has been approved for the treatment of various conditions such as Cluster Headache, Migraine Disorders, Migraine With Aura, and Migraine Without Aura. The drug was first approved in the United States in April 1946.
Dihydroergotamine Mesylate is developed and marketed by Bausch Health Cos., Inc., an originator organization in the pharmaceutical industry. As a small molecule drug, it is characterized by its relatively low molecular weight and ability to penetrate cell membranes, allowing it to interact with specific receptors in the body.
The drug's primary target, the 5-HT1D receptor, is a subtype of the serotonin receptor found in the central and peripheral nervous systems. By binding to this receptor, Dihydroergotamine Mesylate exerts its therapeutic effects, which include vasoconstriction and inhibition of neurogenic inflammation. These actions help alleviate the symptoms associated with Cluster Headache and Migraine Disorders.
Cluster Headache is a severe form of headache characterized by recurrent episodes of intense pain, usually on one side of the head. Migraine Disorders, on the other hand, encompass a broader range of conditions that involve recurrent headaches, often accompanied by other symptoms such as nausea, sensitivity to light, and visual disturbances. Migraine With Aura refers to migraines that are preceded by specific neurological symptoms, while Migraine Without Aura refers to migraines without such preceding symptoms.
Since its first approval in 1946, Dihydroergotamine Mesylate has been widely used in the treatment of these conditions. As a result, it has become an established treatment option for patients suffering from Cluster Headache and various forms of Migraine Disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Dihydroergotamine Mesylate: 5-HT1D receptor agonists
5-HT1D receptor agonists are a type of drugs that activate the 5-HT1D receptors in the body. The 5-HT1D receptors, also known as serotonin 1D receptors, are a subtype of serotonin receptors found in the central nervous system. When these receptors are activated by agonists, they produce various physiological effects.
From a biomedical perspective, 5-HT1D receptor agonists are commonly used in the treatment of migraines. By activating the 5-HT1D receptors, these drugs help to constrict blood vessels in the brain and inhibit the release of certain chemicals that are involved in the development of migraines. This can effectively reduce the frequency and severity of migraine attacks.
Examples of 5-HT1D receptor agonists include sumatriptan, zolmitriptan, and rizatriptan. These drugs are typically administered orally, as nasal sprays, or as injections for acute migraine relief. They are considered a specific class of medications targeting the 5-HT1D receptors and are specifically designed to alleviate migraine symptoms.
It's important to note that the use of 5-HT1D receptor agonists should be done under the guidance of a healthcare professional, as they may have potential side effects and contraindications. Additionally, individual responses to these drugs may vary, and they may not be suitable for everyone.
Drug Target R&D Trends for Dihydroergotamine Mesylate
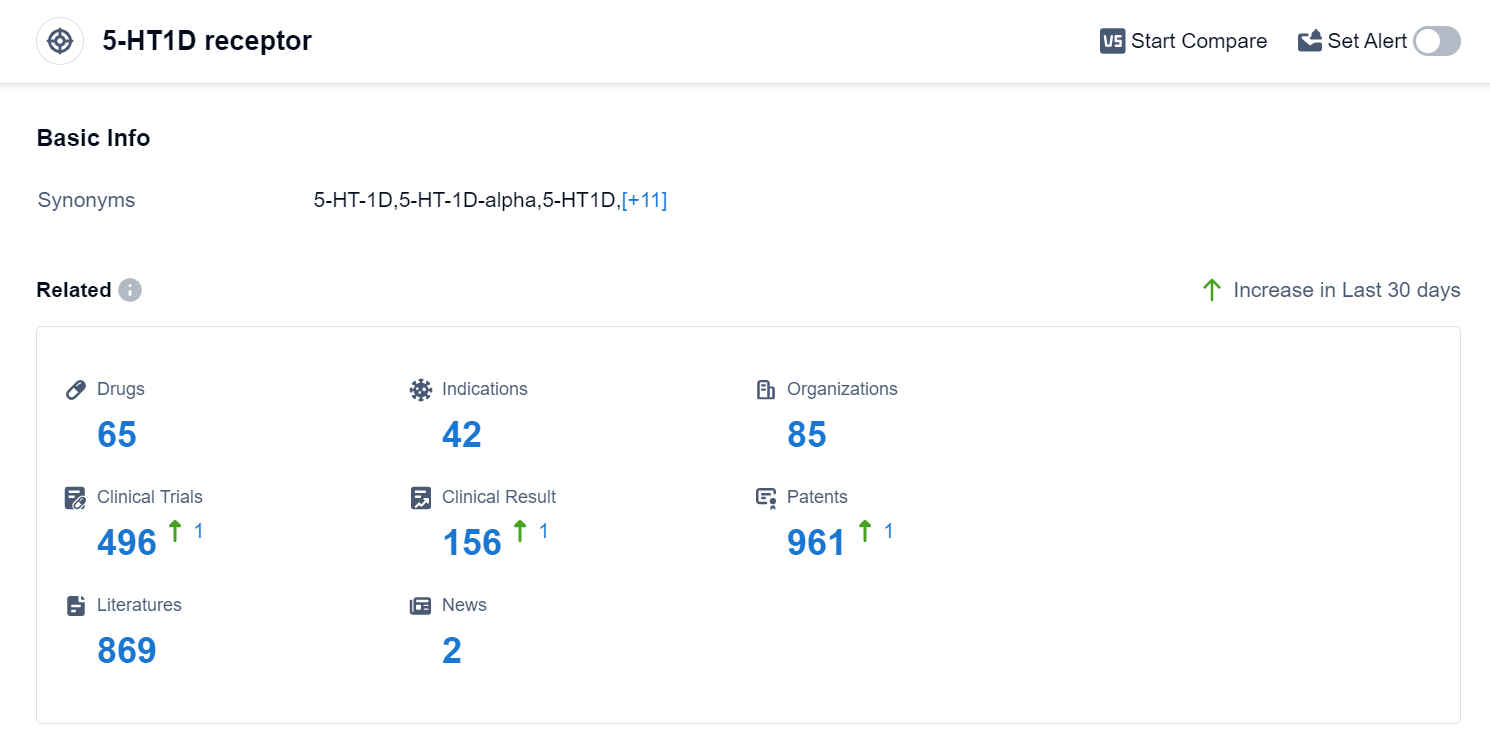
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 65 5-HT1D receptor drugs worldwide, from 85 organizations, covering 42 indications, and conducting 496 clinical trials.
The analysis of the target 5-HT1D receptor reveals that GSK Plc, SAWAI GROUP HOLDINGS Co., Ltd., Eisai Co., Ltd., Pfizer Inc., UCB SA, Merck & Co., Inc., Lundbeck Foundation, Takeda Pharmaceutical Co., Ltd., Endo International Plc, Teva Pharmaceutical Industries Ltd., and Grupo Corporativo Landon SL are the companies growing fastest under this target. The highest stage of development is the "Approved" phase, with a focus on indications such as Migraine Disorders, Cluster Headache, and Depressive Disorder. Small molecule drugs are progressing most rapidly, and the United States, European Union, and China are the countries developing fastest under this target. The future development of the target 5-HT1D receptor is expected to continue with a focus on these companies, indications, drug types, and countries/locations.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Dihydroergotamine Mesylate is a small molecule drug developed by Bausch Health Cos., Inc. It targets the 5-HT1D receptor and is approved for the treatment of Cluster Headache, Migraine Disorders, Migraine With Aura, and Migraine Without Aura. Its first approval dates back to 1946 in the United States, and it has since gained recognition as an effective therapeutic option for patients with these conditions.