Exploring Ibrutinib: A Comprehensive Overview of Applications, Regulatory Approvals, and Advanced Synthesis Processes
Ibrutinib is a medication used primarily in the treatment of certain types of cancer, particularly B-cell malignancies such as chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and Waldenström's macroglobulinemia. It is marketed under the brand name Imbruvica, among others.Ibrutinib belongs to a class of drugs known as Bruton's tyrosine kinase (BTK) inhibitors. It works by blocking BTK, a key enzyme in the B-cell receptor signaling complex, which plays an essential role in the survival and proliferation of B cells. By inhibiting BTK, ibrutinib can help to kill cancer cells and prevent their growth.
This medication is typically used for patients who have received at least one prior therapy, or as a first-line treatment in specific circumstances. The effectiveness of ibrutinib has made it a significant advancement in the treatment of these cancers.Ibrutinib is administered orally and can have various side effects, including diarrhea, bleeding, fatigue, and increased risk of infections, among others. Due to its targeted mode of action, it is generally better tolerated than traditional chemotherapy, although serious complications can still occur.
The drug was developed by Pharmacyclics LLC and has obtained the highest phase of approval in both global and China markets. Ibrutinib received its first approval in the United States in November 2013, with various regulatory designations including priority review, accelerated approval, fast track, breakthrough therapy, orphan drug, and special review project.
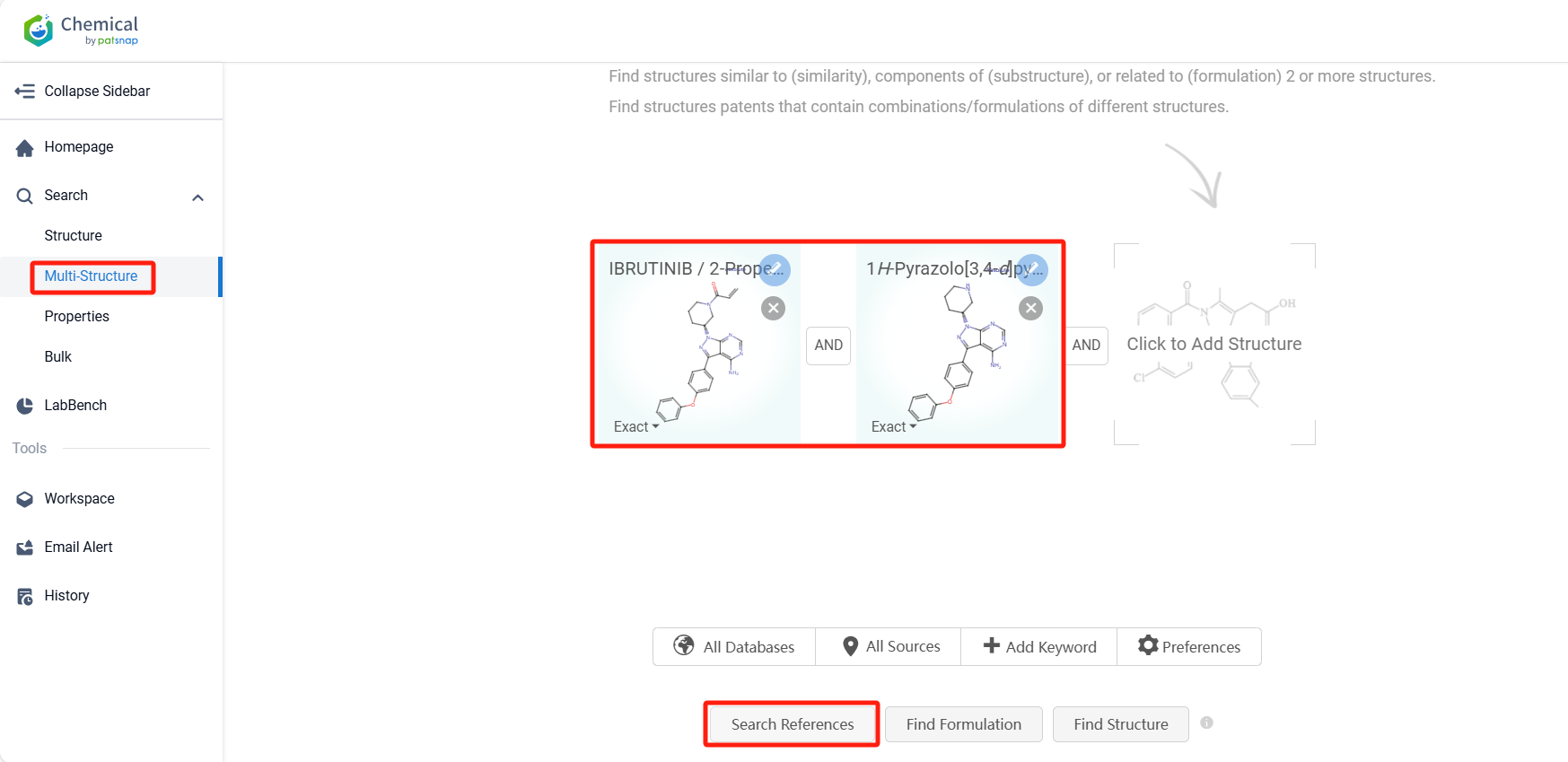
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Ibrutinib. click on search references, and you can query the relevant literature.
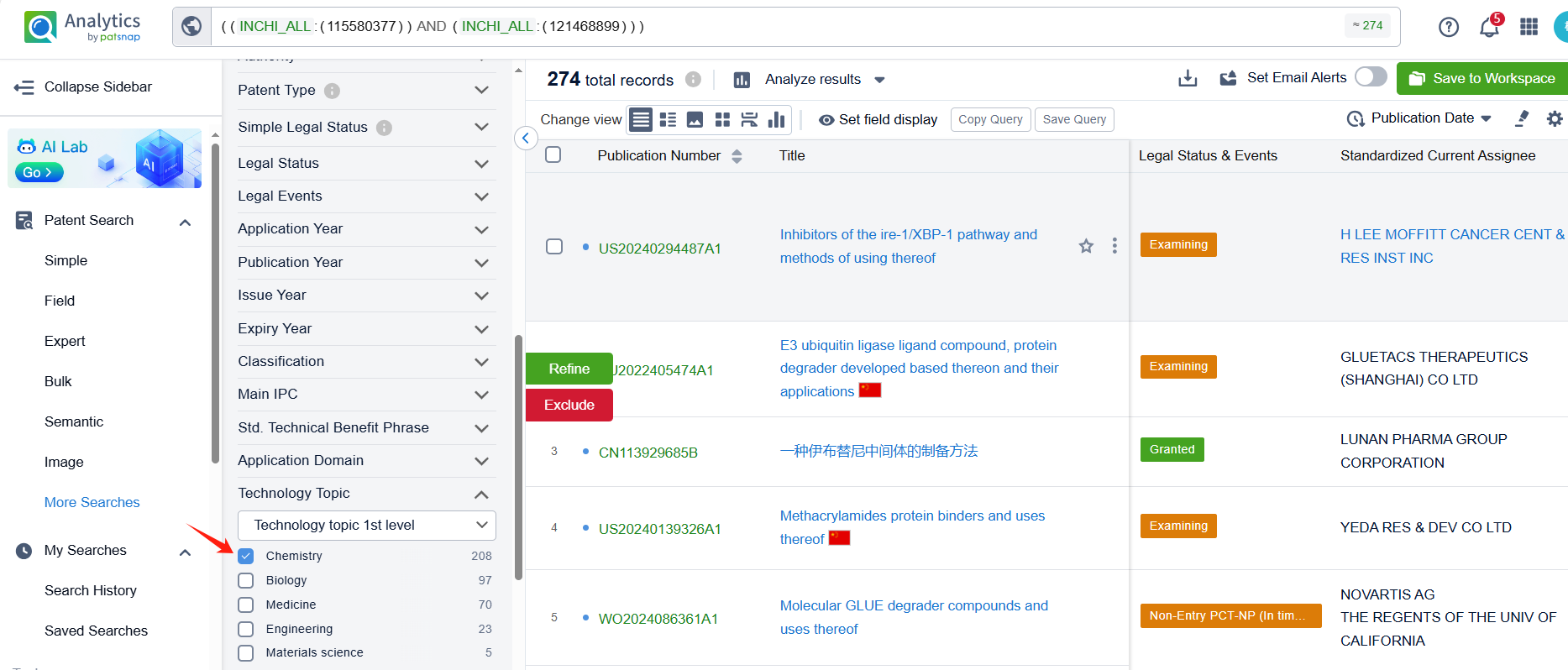
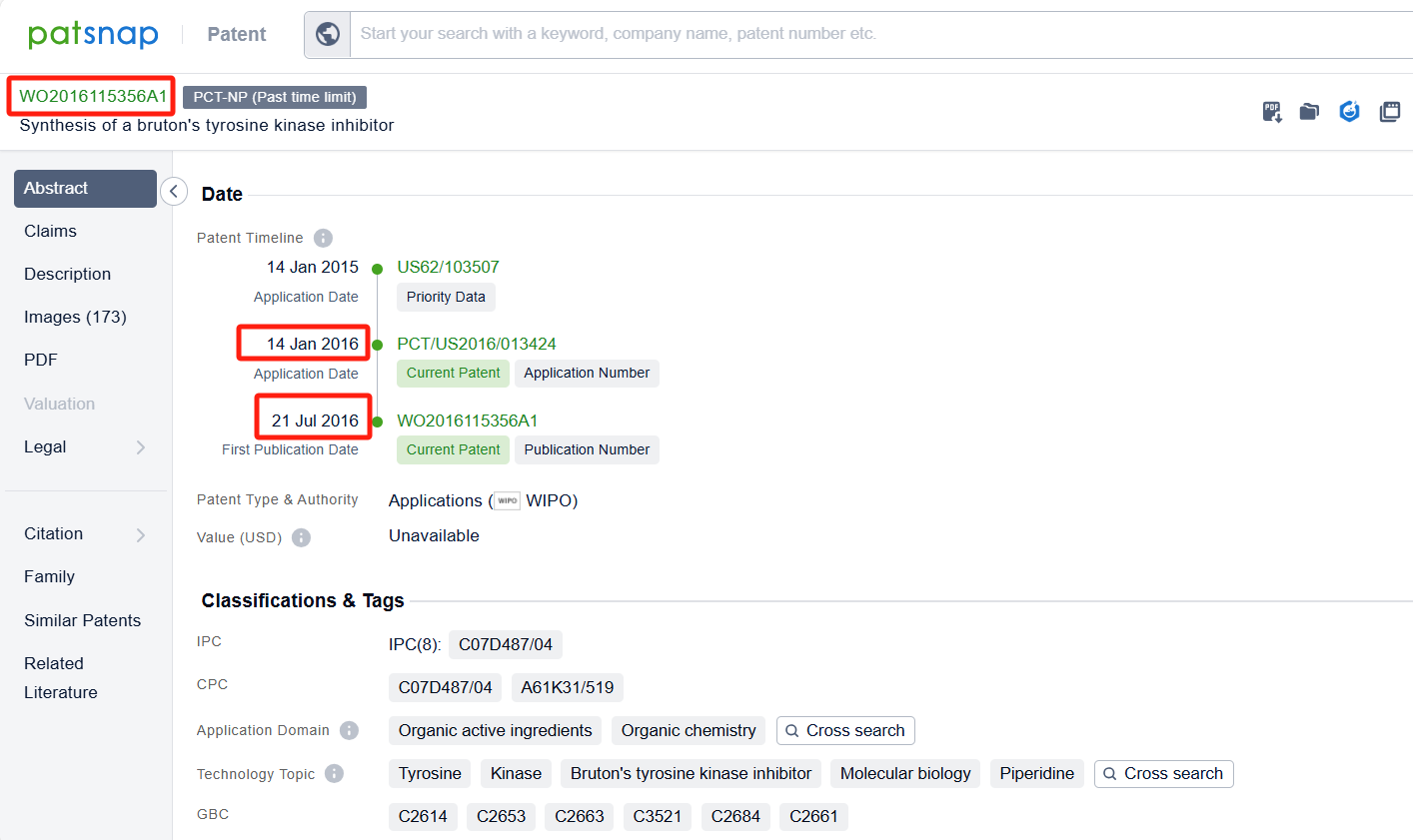
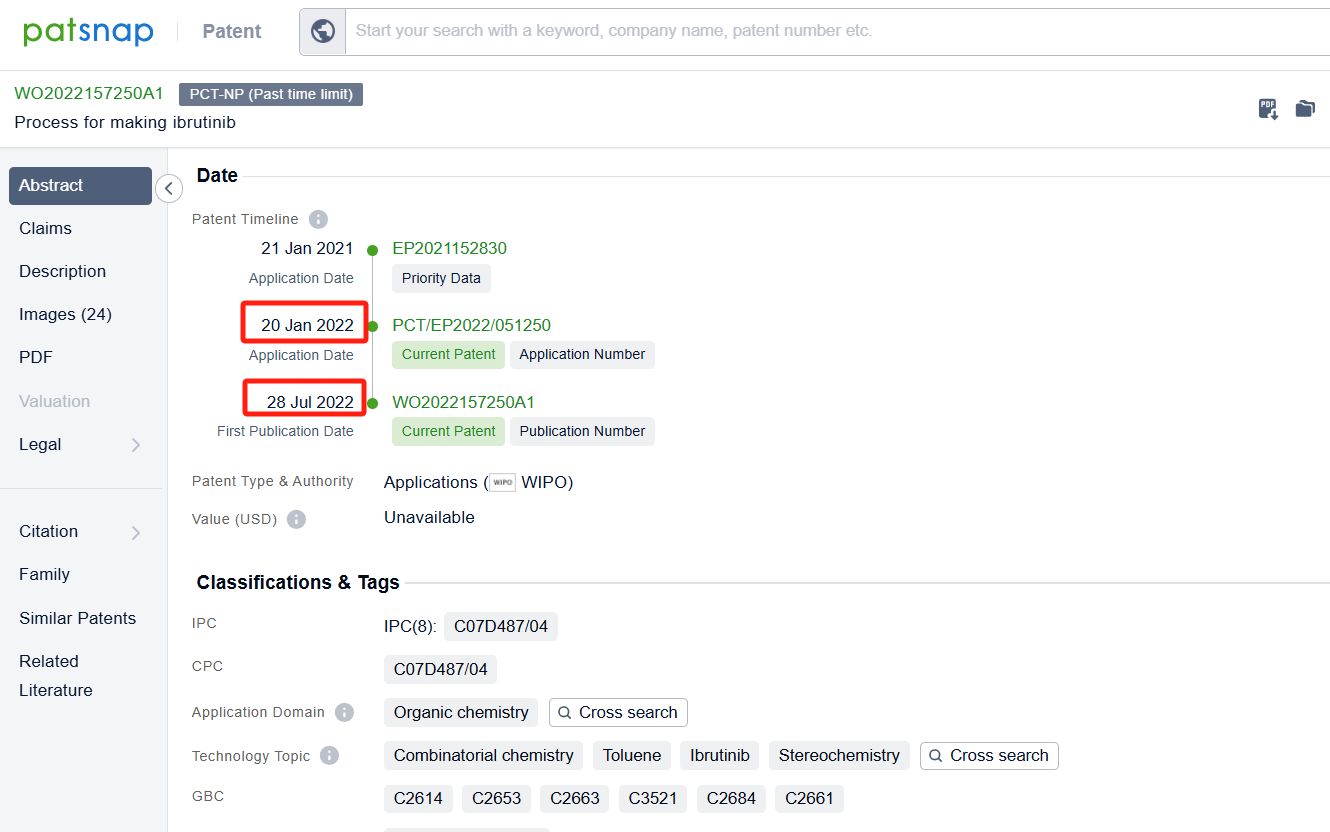
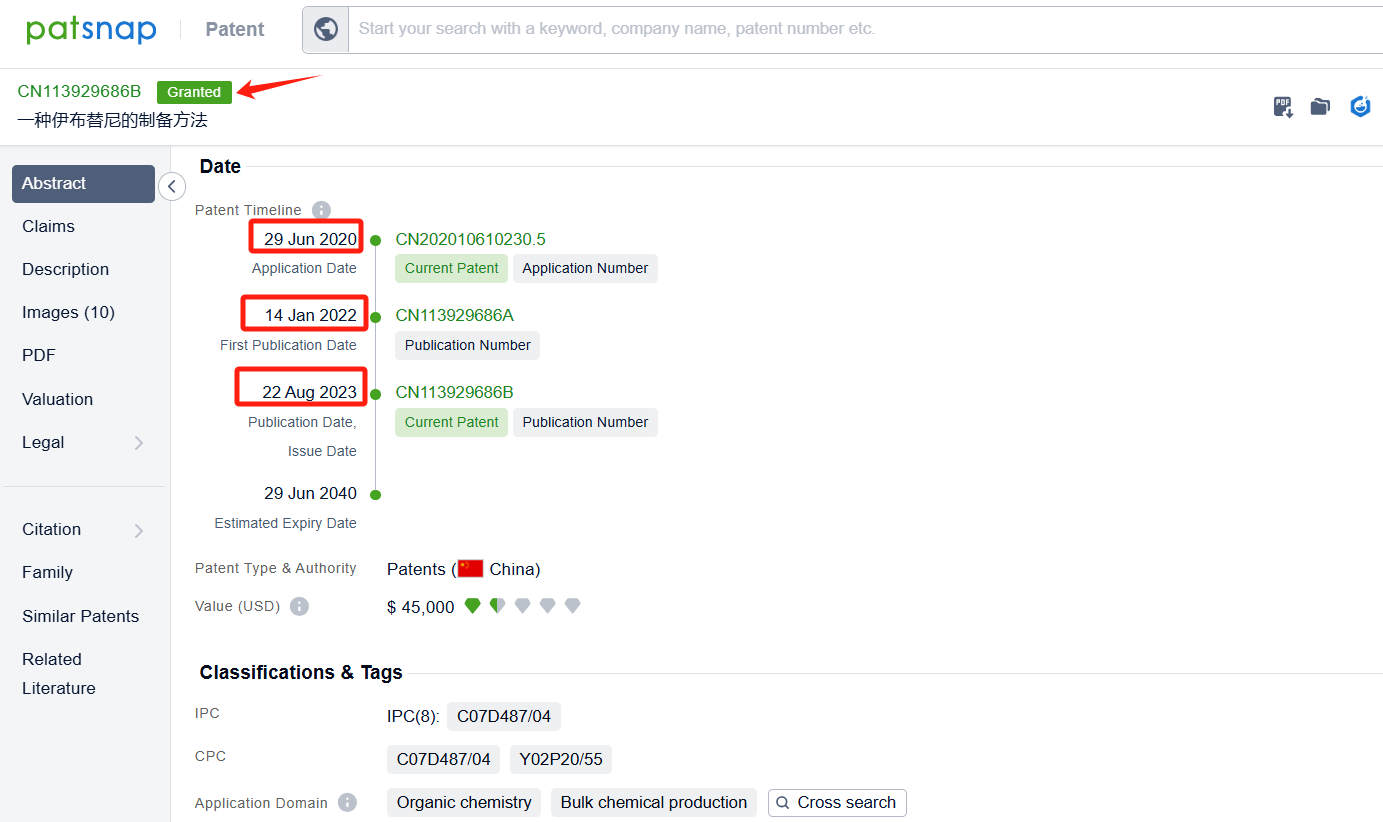
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Ibrutinib process route by Pharmacyclics LLC and other companies,Such as Pharmacyclics LLC's international patent WO2016115356A1 (application date 20160114, publication date 20160721) provide a more efficient and effective method for making ibrutinib. Synthon BV's international patent WO2022157250A1 (application date 20220120, publication date 20220728) describes a process for making Ibrutinib. The process involves combining two different chemicals, using a special catalyst, and then isolating a solid form of the resulting compound. Finally, the solid compound is transformed into Ibrutinib. Additionally, Lunan Pharmaceutical Group Co., Ltd.'s patent CN113929686A (application date 20200629, publication date 20220114) provides a new method for preparing ibrutinib. By using an aromatic aldehyde to disulfide and then introducing diphenyl ether through Mannich reaction, it avoids the traditional Suzuki coupling reaction with low yield and expensive catalyst. The patent was granted on August 22, 2023.
In summary, Ibrutinib is a potent small molecule drug targeting BTK with a broad spectrum of therapeutic areas and active indications. It has received approvals in multiple countries and has been granted various regulatory designations to expedite its development and approval process. As an expert in the pharmaceutical industry, it is essential to continually monitor and analyze the progress and impact of Ibrutinib in the market to identify potential business development opportunities and partnerships.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.