Viking Therapeutics Shares Fresh Findings on VK2735 Obesity Program at ObesityWeek® 2024
Viking Therapeutics, Inc. (NASDAQ: VKTX), a biopharmaceutical firm in the clinical development stage that specializes in creating innovative treatments for metabolic and endocrine disorders, has announced that new clinical findings from its VK2735 obesity initiative were featured in two poster sessions at ObesityWeek® 2024, the annual conference organized by the Obesity Society. VK2735 functions as a dual agonist for the glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors and is being investigated for its potential in treating various metabolic conditions. The company is exploring both subcutaneous and oral delivery methods for VK2735 in its clinical studies.
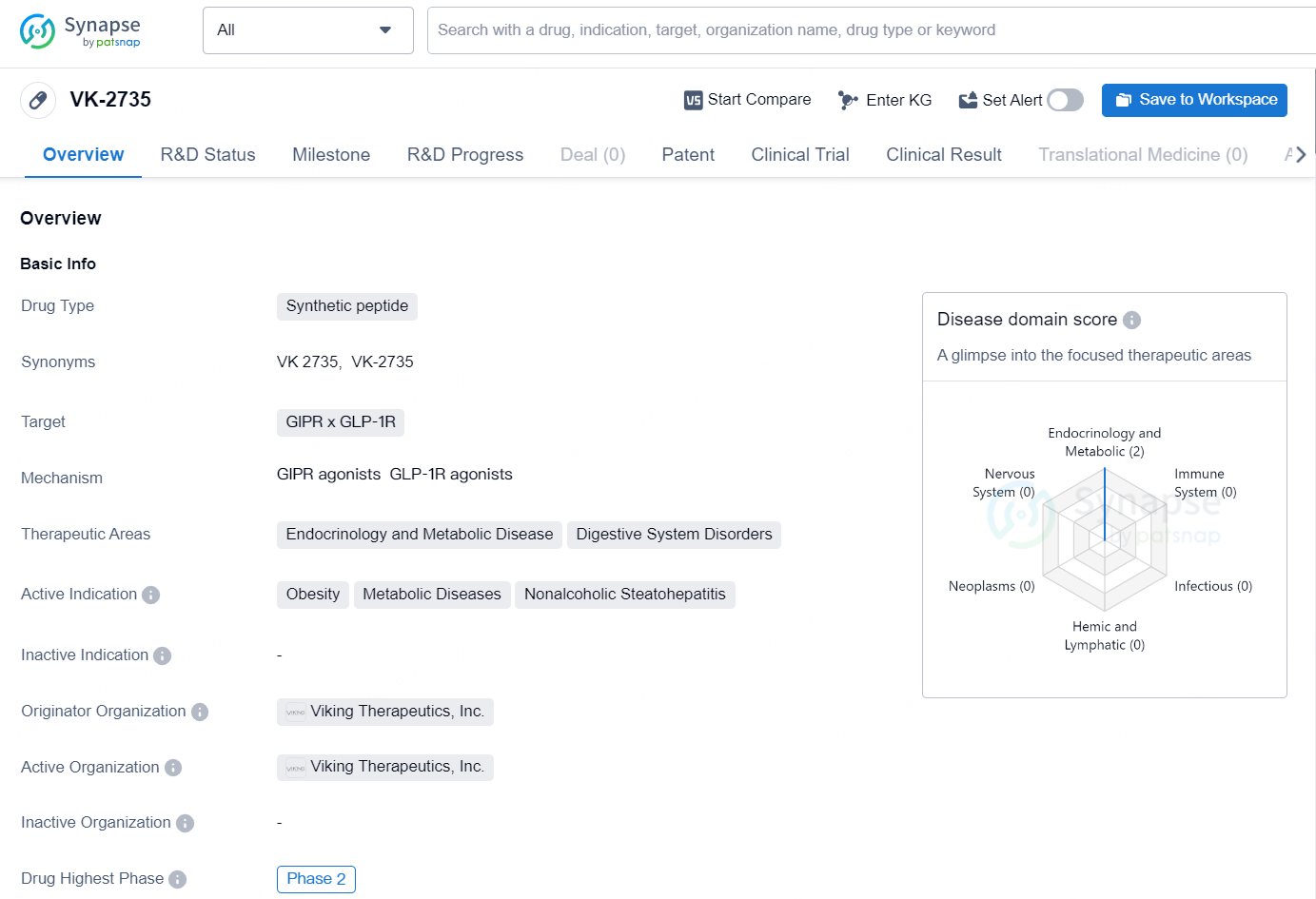
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Key takeaways from one poster presentation include fresh findings from the company's Phase 1 multiple ascending dose (MAD) clinical trial involving an oral tablet formulation of VK2735 administered daily over 28 days. This presentation covers results from the treatment groups receiving daily doses of 60 mg, 80 mg, and 100 mg. Another poster featured follow-up data from the company's Phase 2 VENTURE clinical trial of VK2735, which illustrated the longer-term maintenance effects of the treatment along with pharmacokinetic data.
Poster #017 presented new findings from the previously disclosed 28-day MAD study on the oral VK2735 tablet, including data from subjects taking 60 mg, 80 mg, and 100 mg daily. The outcomes continued to indicate encouraging signs of clinical efficacy throughout the treatment duration. Participants treated with VK2735 exhibited dose-dependent decreases in mean body weight from the baseline, with reductions reaching as high as 8.2%. Additionally, VK2735 cohorts showed decreases in mean body weight compared to placebo, peaking at 6.8%. Notably, ongoing weight loss effects were recorded during follow-up visits until Day 57, with reductions up to 8.3% from baseline four weeks post the last VK2735 dose. An exploratory analysis indicated that nearly 100% of VK2735 patients achieved at least a 5% reduction in weight after the 28-day period, while the placebo group reported 0% success. Preliminary assessments of weight loss patterns at various dosing levels suggest that extending treatment beyond 28 days may result in further reductions in body weight.
Poster #018 provided updated findings from follow-up evaluations conducted in the previously disclosed 13-week Phase 2 VENTURE study that examined VK2735 as a subcutaneous injection. As noted earlier, patients who received weekly VK2735 doses exhibited statistically significant declines in mean body weight after 13 weeks, reaching up to 14.7% from their baseline measurements. Statistically significant differences were seen when comparing both baseline and placebo results starting from Week One and persisting through the entire 13-week study. Weight loss was progressive throughout the trial, with no weight loss plateau observed at the 13-week mark. Furthermore, as much as 88% of patients in the VK2735 treatment groups achieved at least a 10% weight reduction, in contrast to just 4% for those receiving placebo.
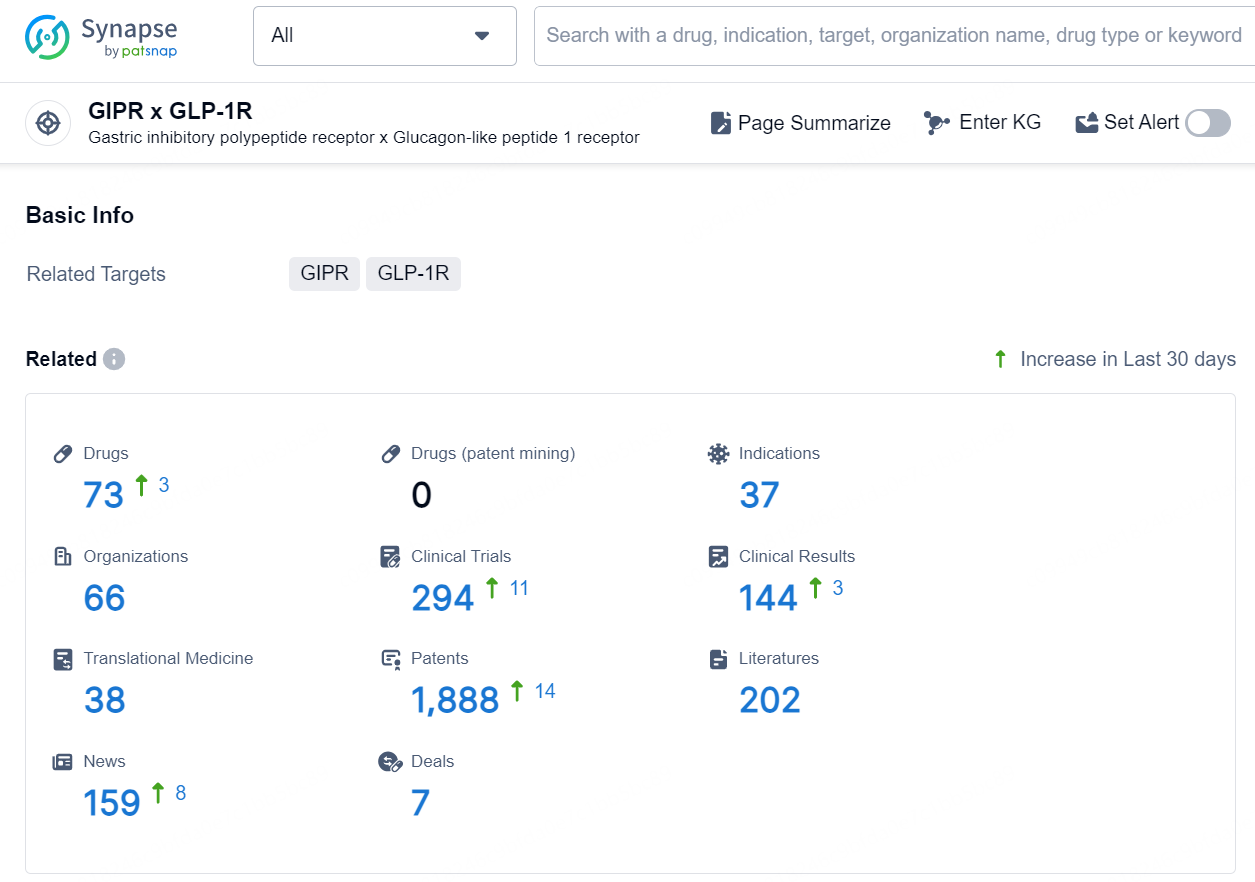
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 5, 2024, there are 73 investigational drugs for the GIPR x GLP-1R target, including 37 indications, 66 R&D institutions involved, with related clinical trial reaching 294, and as many as 1888 patents.
The drug VK-2735 is a synthetic peptide designed to target GIPR and GLP-1R receptors in the body. It is primarily focused on treating endocrinology and metabolic diseases, as well as digestive system disorders. The active indication for this drug includes obesity, metabolic diseases, and nonalcoholic steatohepatitis.