Exploring teniposide's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Teniposide's R&D Progress
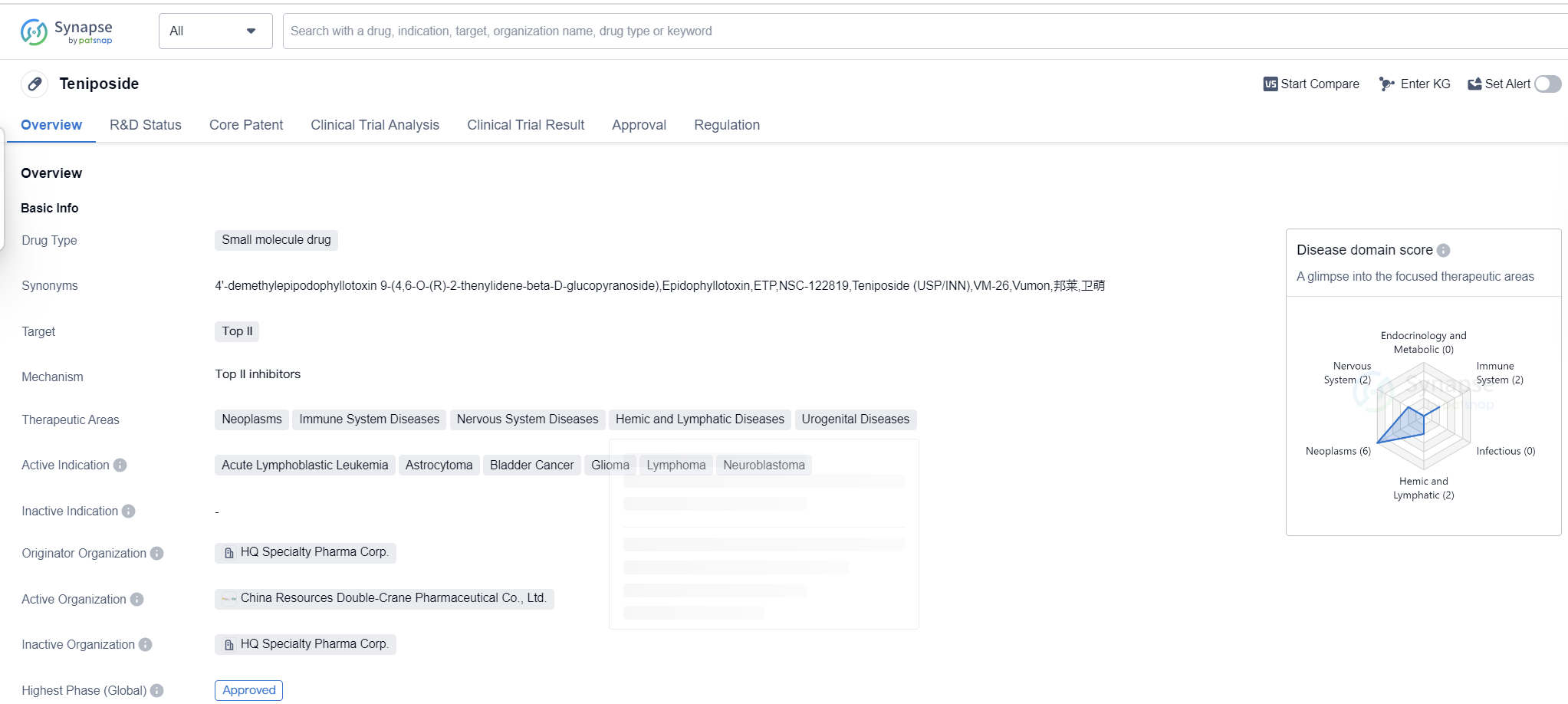
Teniposide is a small molecule drug that falls under the category of biomedicine. It primarily targets Top II, making it suitable for the treatment of various diseases. The drug has shown potential in therapeutic areas such as neoplasms, immune system diseases, nervous system diseases, hemic and lymphatic diseases, and urogenital diseases.
Teniposide has been found to be effective in treating several active indications, including acute lymphoblastic leukemia, astrocytoma, bladder cancer, glioma, lymphoma, and neuroblastoma. These indications highlight the drug's versatility and potential to address different types of cancers and tumors.
The drug was developed by HQ Specialty Pharma Corp., an originator organization in the pharmaceutical industry. It has reached the highest phase of development which is approved globally. The global approval for Teniposide was granted in July 1992, with the United States being the first country to approve its use.
Teniposide is regulated as an orphan drug, indicating that it is intended to treat rare diseases or conditions. This regulatory status highlights the drug's potential to address unmet medical needs and provide therapeutic options for patients with limited treatment options.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for teniposide: Top II inhibitors
Top II inhibitors refer to a class of compounds or drugs that inhibit the activity of the enzyme topoisomerase II. Topoisomerase II is an essential enzyme involved in DNA replication, transcription, and repair. It plays a crucial role in maintaining the integrity and structure of DNA by regulating the topological changes in DNA strands.
In the context of biomedicine, top II inhibitors are of significant interest as potential anticancer agents. Cancer cells often have increased DNA replication and transcription rates, making them more dependent on topoisomerase II activity. By inhibiting this enzyme, top II inhibitors can disrupt DNA replication and transcription in cancer cells, leading to their death or growth inhibition.
Top II inhibitors can be classified into two main types: topoisomerase II poisons and catalytic inhibitors. Topoisomerase II poisons, such as etoposide and doxorubicin, stabilize the enzyme-DNA complex, preventing the DNA strands from resealing and causing DNA breaks. Catalytic inhibitors, on the other hand, interfere with the catalytic activity of topoisomerase II without causing DNA breaks.
The development and use of top II inhibitors in cancer treatment have shown promising results, particularly in hematological malignancies and solid tumors. However, these inhibitors can also have side effects due to their impact on normal cells that rely on topoisomerase II activity. Therefore, their use requires careful consideration and monitoring to ensure optimal therapeutic benefits while minimizing adverse effects.
Drug Target R&D Trends for teniposide
Top II, also known as topoisomerase II, is an essential enzyme found in the human body that plays a crucial role in DNA replication and repair. It is responsible for managing the topological changes in DNA structure by introducing temporary breaks in the DNA strands. This allows for the unwinding and separation of DNA during replication and transcription processes. Top II is involved in regulating the supercoiling of DNA, ensuring its proper functioning and stability. Additionally, this enzyme is a target for certain pharmaceutical drugs, as inhibiting its activity can disrupt DNA replication and lead to cell death, making it an important target for cancer treatments.
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 256 Top II drugs worldwide, from 298 organizations, covering 189 indications, and conducting 4755 clinical trials.
The analysis of the current competitive landscape and future development of target Top II reveals that multiple companies are actively involved in the research and development of drugs under this target. Pfizer Inc. has the highest number of approved drugs, indicating its strong presence in the market. The indications with the highest number of approved drugs include leukemia, lymphoma, breast cancer, and acute myeloid leukemia. Small molecule drugs are progressing rapidly, along with the development of biosimilars such as ADCs and monoclonal antibodies. China, the European Union, Japan, and the United States are the leading countries in terms of drug development. China, in particular, has made significant progress in its research and development efforts. Overall, the target Top II presents a competitive landscape with multiple companies and a focus on specific indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Teniposide is a small molecule drug developed by HQ Specialty Pharma Corp. It targets Top II and has shown efficacy in treating various diseases, particularly in the field of oncology. With approved indications for acute lymphoblastic leukemia, astrocytoma, bladder cancer, glioma, lymphoma, and neuroblastoma, Teniposide offers potential therapeutic benefits for patients. Its orphan drug status further emphasizes its importance in addressing unmet medical needs.