Exploring the Latest CAR-T therapy Deal by 2seventy Bio: A Guide to Rapidly Accessing Transaction Insights
On January 30, 2024, Regeneron announced that, under an agreement with 2seventy bio, Regeneron will acquire the rights to develop and commercialize 2seventy bio’s entire pipeline of novel immuno-cell therapies, as well as its discovery and clinical manufacturing capabilities. In addition, 270 biotechnology employees working on the 2seventy bio projects will join Regeneron’s newly established R&D division, Regeneron Cell Medicines, to advance the development of the acquired projects as well as Regeneron’s own cell therapy projects.
According to the terms of the agreement, Regeneron will pay 2seventy bio an upfront payment of $5 million, and upon the first product from the transaction receiving market approval, Regeneron will make a milestone payment to 2seventy bio. The transaction is expected to be completed in the first half of 2024.
Previously, in 2018, Regeneron and bluebird bio (which later spun off 2seventy bio in 2021) had reached an agreement to develop new cell therapies for cancer using the complementary technologies of both companies. Under the original agreement, Regeneron had the option to develop/commercialize arrangements targeting the collaboration goals.
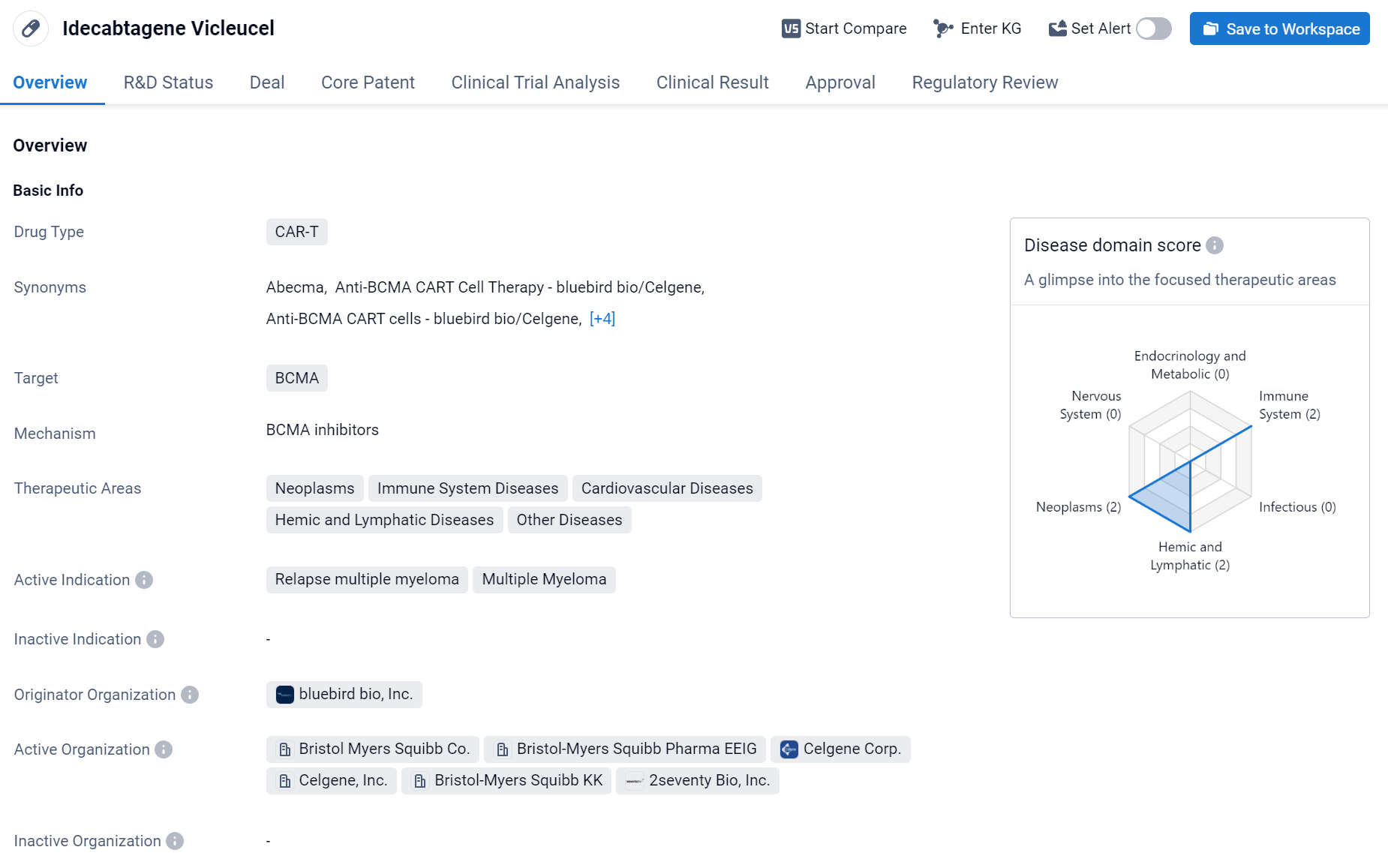
About Idecabtagene Vicleucel
Idecabtagene Vicleucel is a CAR-T therapy drug developed by bluebird bio, Inc. It has been approved for the treatment of relapsed multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. The drug targets BCMA, a protein that is highly expressed on the surface of multiple myeloma cells. Click the image below to directly embark on the exploration journey with the Idecabtagene Vicleucel!
CAR-T therapy is a type of immunotherapy that involves modifying a patient's own T-cells to express a receptor that recognizes and binds to a specific protein on cancer cells. Once the modified T-cells are infused back into the patient, they can recognize and kill cancer cells expressing the target protein.
In March 2021, Abecma was approved by the U.S. FDA for the treatment of relapsed/refractory multiple myeloma (r/r MM). Abecma is priced at $419,500 per bag, with industry projections estimating its peak sales to reach $629 million.
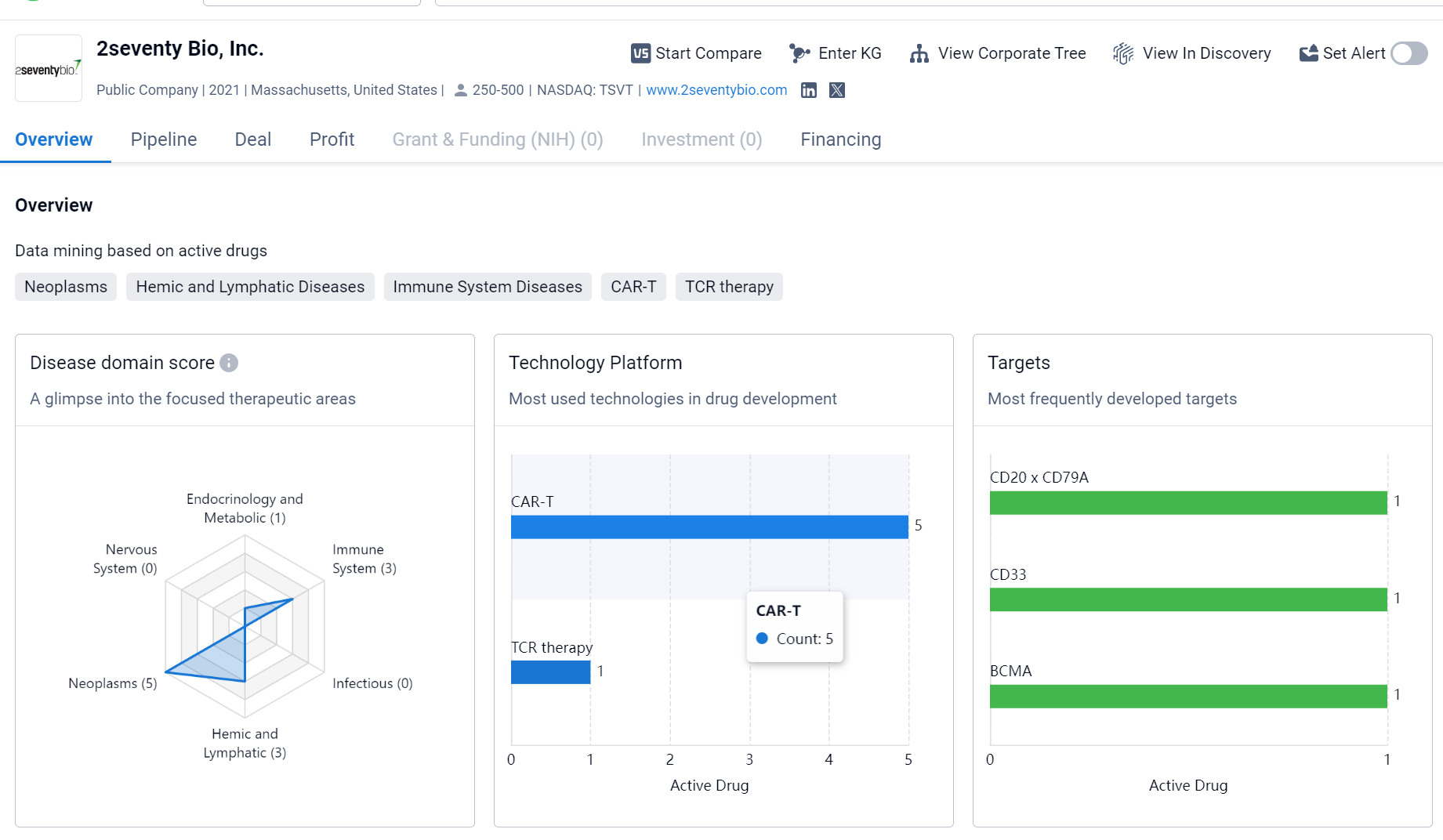
About 2seventy Bio
2seventy Bio, Inc. is a relatively new biomedicine organization based in Massachusetts, United States. The company has a diverse pipeline of drugs targeting various therapeutic areas, with a particular focus on Neoplasms, Hemic and Lymphatic Diseases, and Immune System Diseases. The most frequently developed targets include CD20 x CD79A, CD33, and BCMA. The pipeline includes drugs in different stages of development, ranging from Discovery to Phase 3. However, the company has not yet reached the IND Application or IND Approval stages, indicating that it is still in the early phases of development.
2seventy bio specializes in CAR-T cell therapies and gene-editing technologies. Additionally, 2seventy bio is also developing gene-editing technologies like the CRISPR-Cas9 system, which is used to precisely modify a patient's genome to treat hereditary diseases and other conditions.
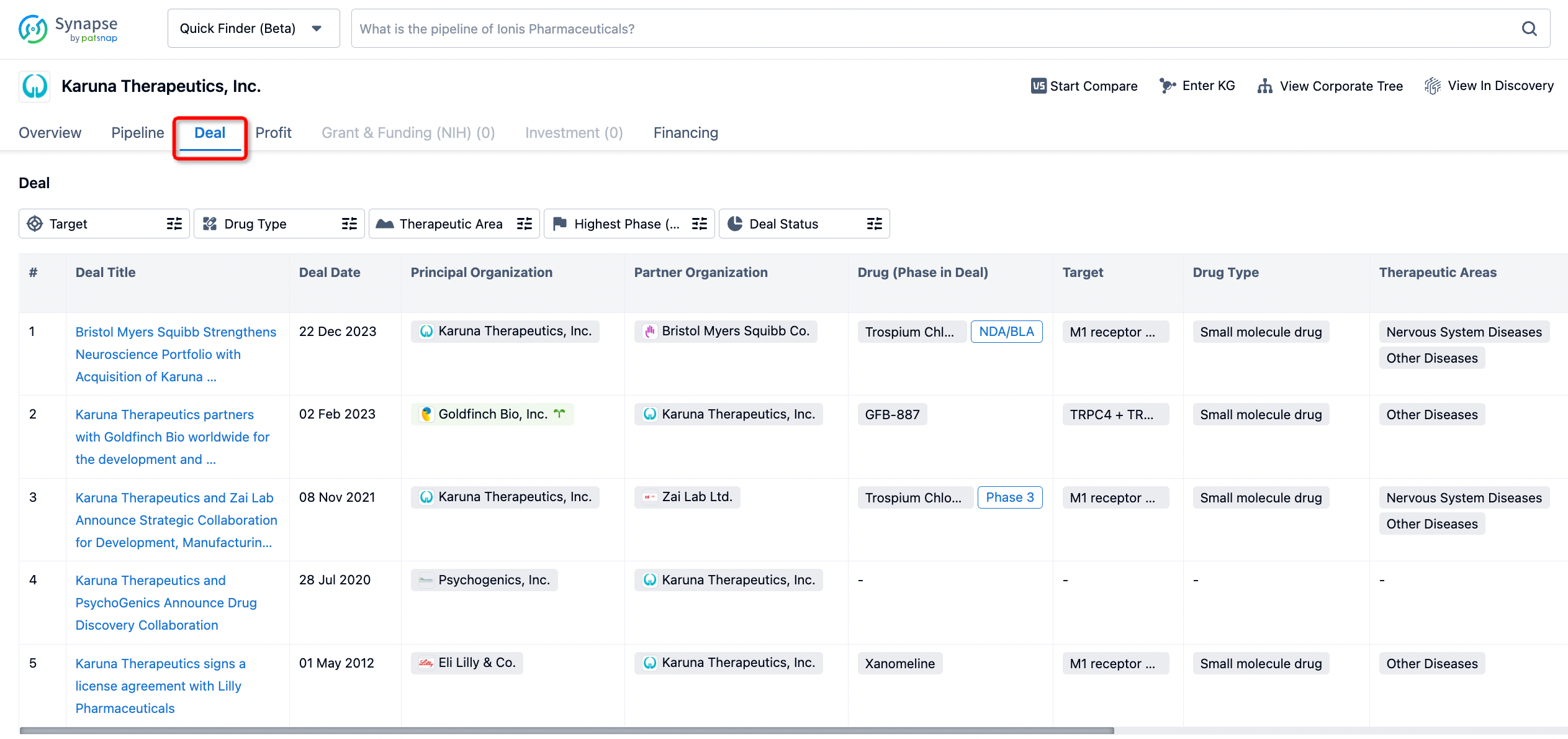
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.

Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
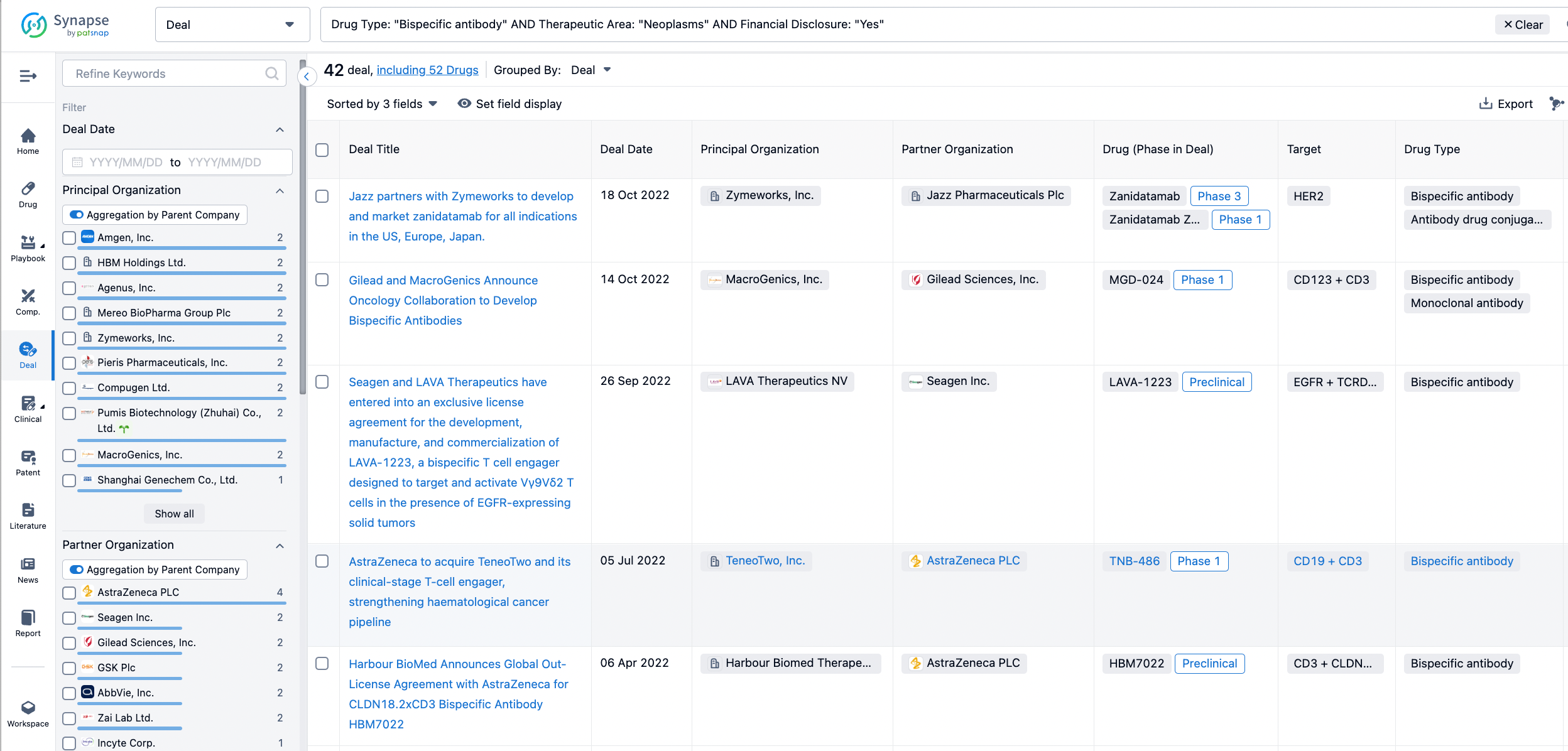
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
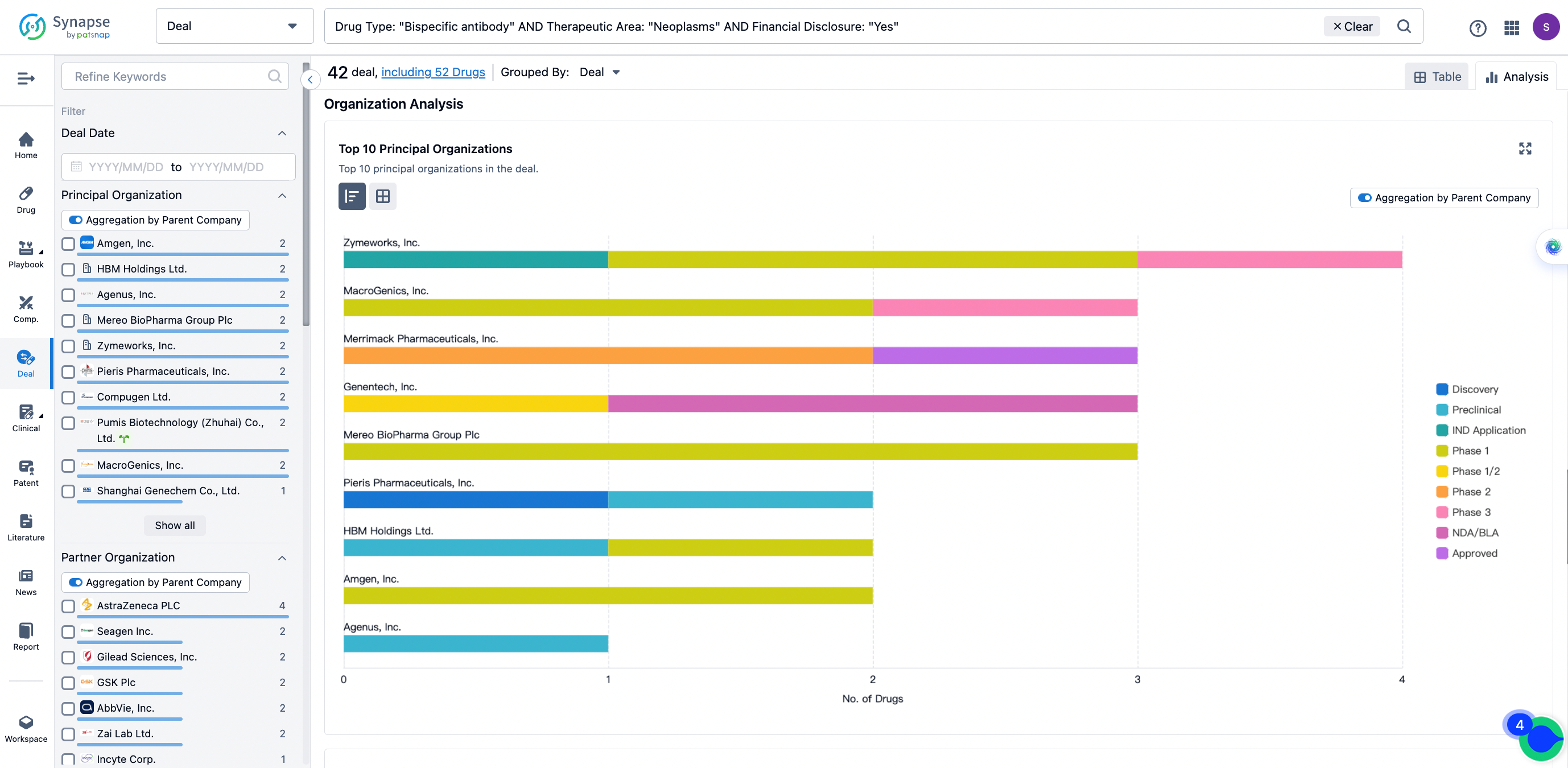
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
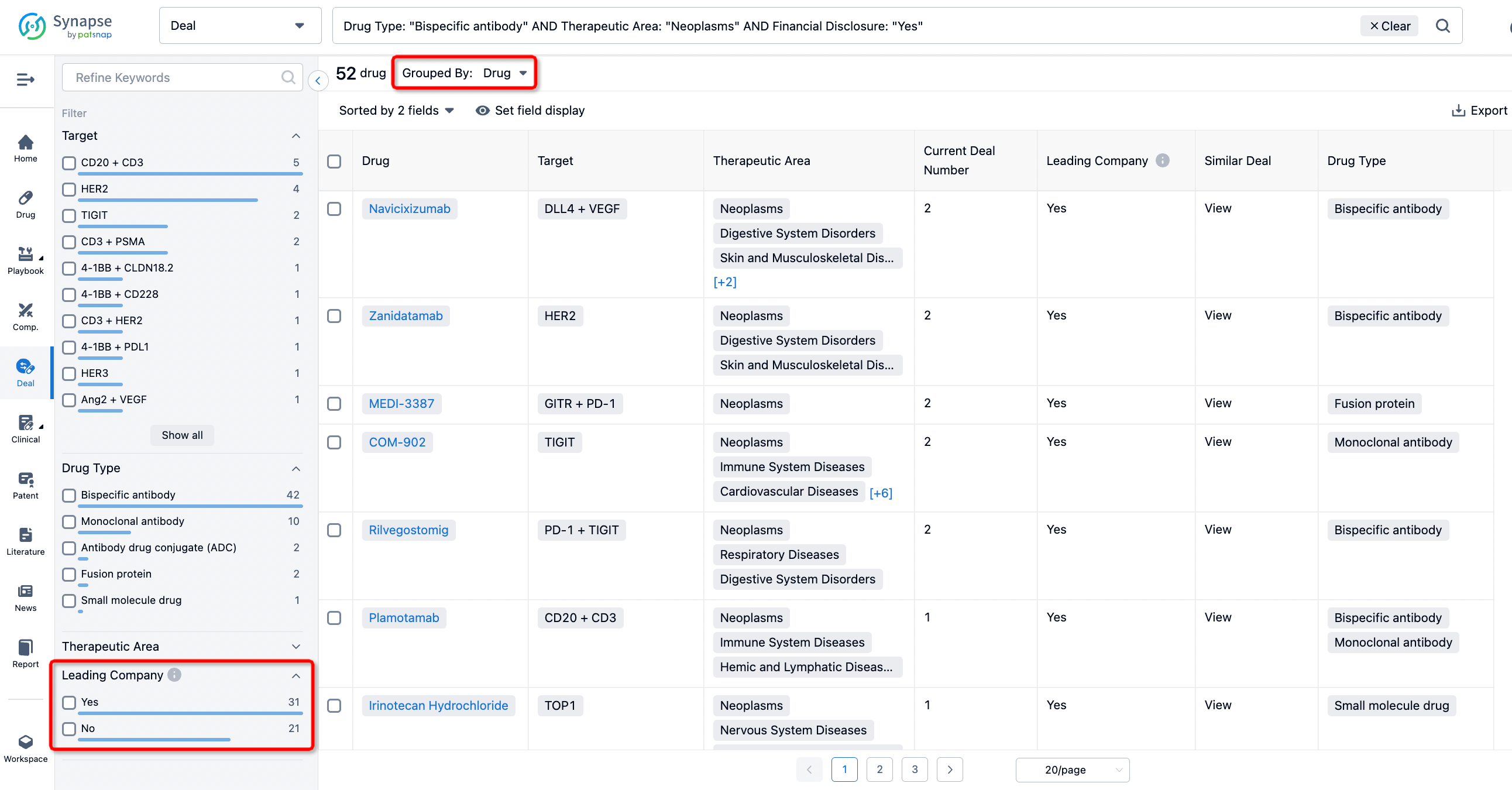
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
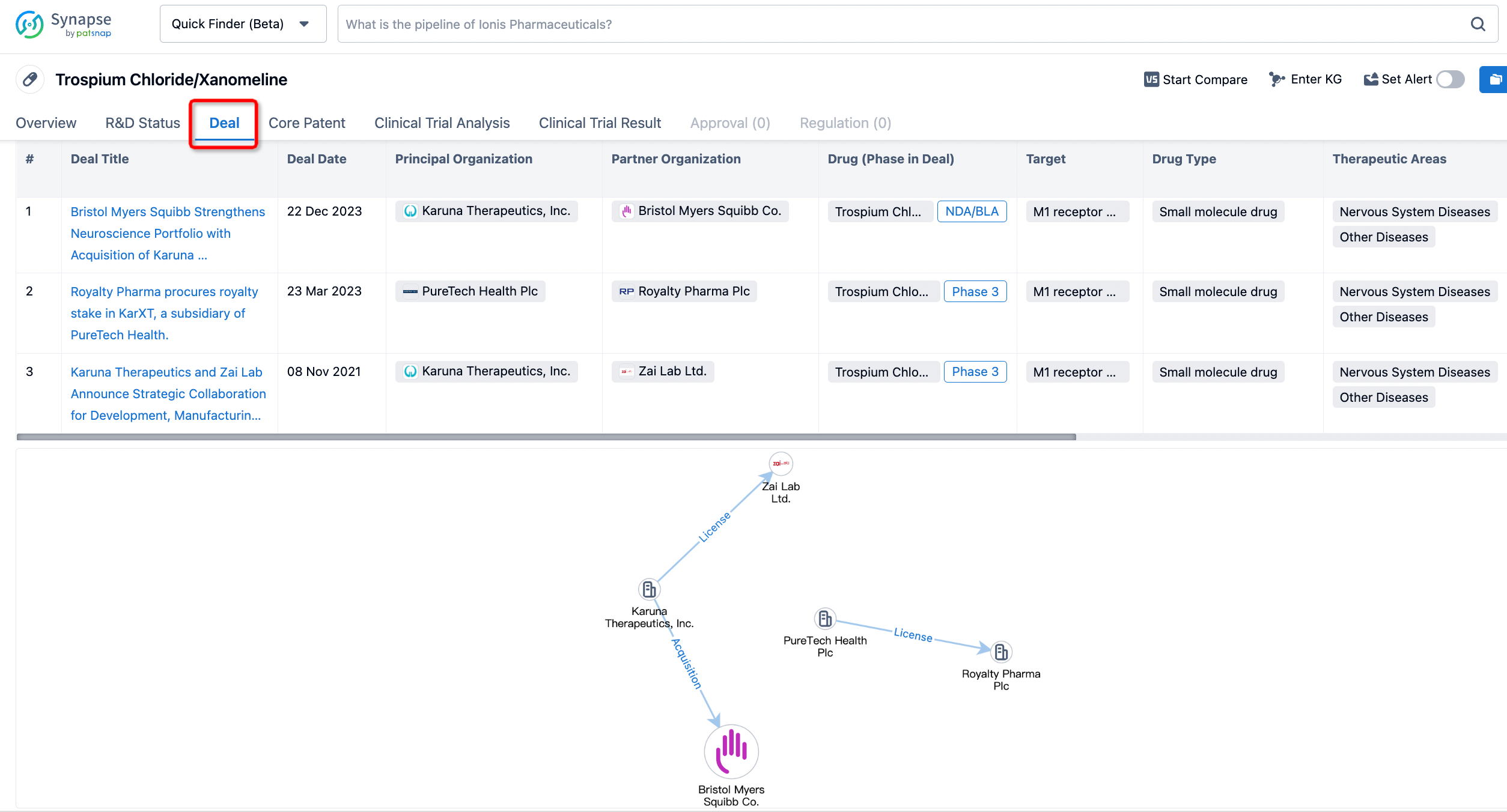
Click on the image below to embark on a brand new journey of drug discovery!