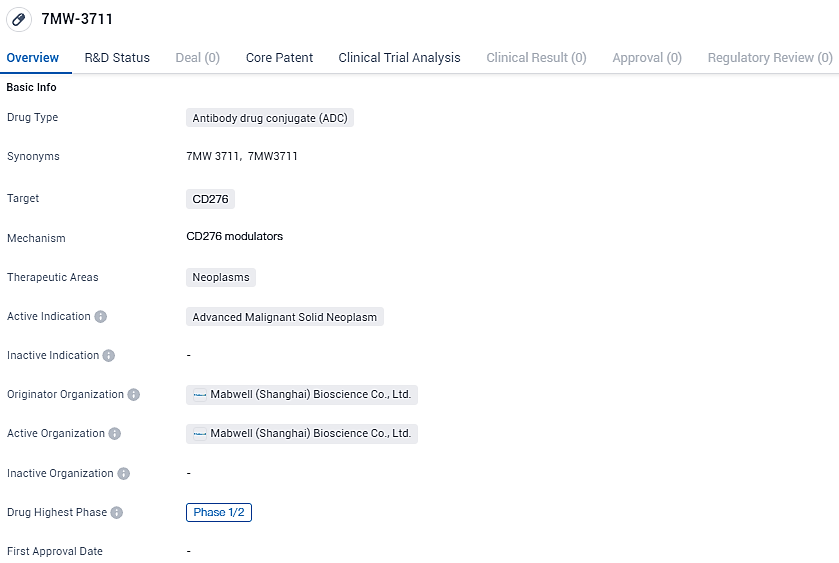
FDA Approves Mabwell's IND Application for B7-H3-Targeted ADC, 7MW3711
Mabwell, a pioneering firm in the biopharmaceutical sector with comprehensive supply chain capabilities, has publicized the sanction of their clinical research proposal for an advanced ADC focusing on B7-H3 (7MW3711) aimed at treating progressive malignant solid neoplasms by the U.S. Food and Drug Administration. The compound, 7MW3711, is a product of Mabwell's cutting-edge IDDC™ antibody-drug conjugate technology platform. The initiation of 7MW3711's clinical studies has commenced within the Chinese clinical trial environment.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The innovative antibody-drug conjugate 7MW3711, which features a unique intellectual property, consists of a cutting-edge antibody, a freshly developed linker, and a newly designed payload. Once administered into the body, 7MW3711 precisely targets the antigen present on the surface of tumor cell membranes, is then internalized, and relocates to the lysosome where it releases a potent cytotoxic agent, ultimately triggering apoptosis in tumor cells.
With its stable and uniform structure and high purity, 7MW3711 stands out for its potential scalability in production. Compared to its international and domestic counterparts, 7MW3711 has demonstrated superior efficacy in eradicating tumors across various animal model studies.
During safety assessments involving animal species such as cynomolgus monkeys, 7MW3711 exhibited a favorable safety profile along with favorable pharmacokinetic attributes. These findings underscore 7MW3711's potential for unique clinical applications and its prospects for robust clinical trials.
Developed by Mabwell, the IDDC™ platform represents the forefront of site-specific ADC technologies. It integrates an array of patented subsystems—such as the precise conjugation method DARfinity™, the custom linker IDconnect™, the innovative payload Mtoxin™, and the specific activation mechanism LysOnly™. This platform enhances ADC products by ensuring uniform structures, stable quality, effective pharmacodynamics, and improved safety profiles.
As of now, the efficacy of the IDDC™ platform has been corroborated through several products that are currently in development. Marking a significant milestone, Mabwell secured approval from China's Center for Drug Evaluation for their Trop-2 targeting ADC product, 9MW2921, in July 2023, and has commenced a clinical trial to address advanced solid tumors. Looking forward, the company anticipates that a series of ADC products will move into clinical trial phases by the year 2024.
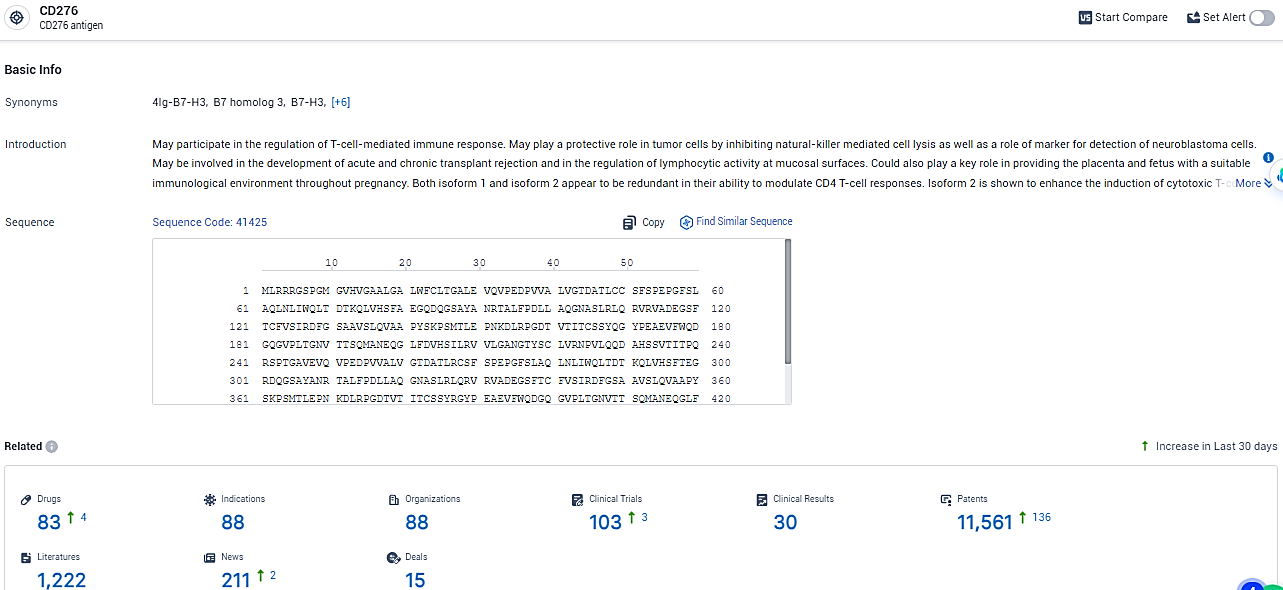
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 26, 2024, there are 83 investigational drugs for the B7-H3 target, including 88 indications, 88 R&D institutions involved, with related clinical trials reaching 103, and as many as 11561 patents.
7MW-3711 combines the targeting ability of an antibody with the cytotoxic effects of a drug. The antibody specifically binds to B7-H3 on cancer cells, delivering the attached drug directly to the tumor cells. This targeted approach aims to minimize damage to healthy cells and increase the drug's effectiveness against cancer.