Exploring the Latest KRAS G12C Inhibitor Deal by Innovent Biologics: A Guide to Rapidly Accessing Transaction Insights
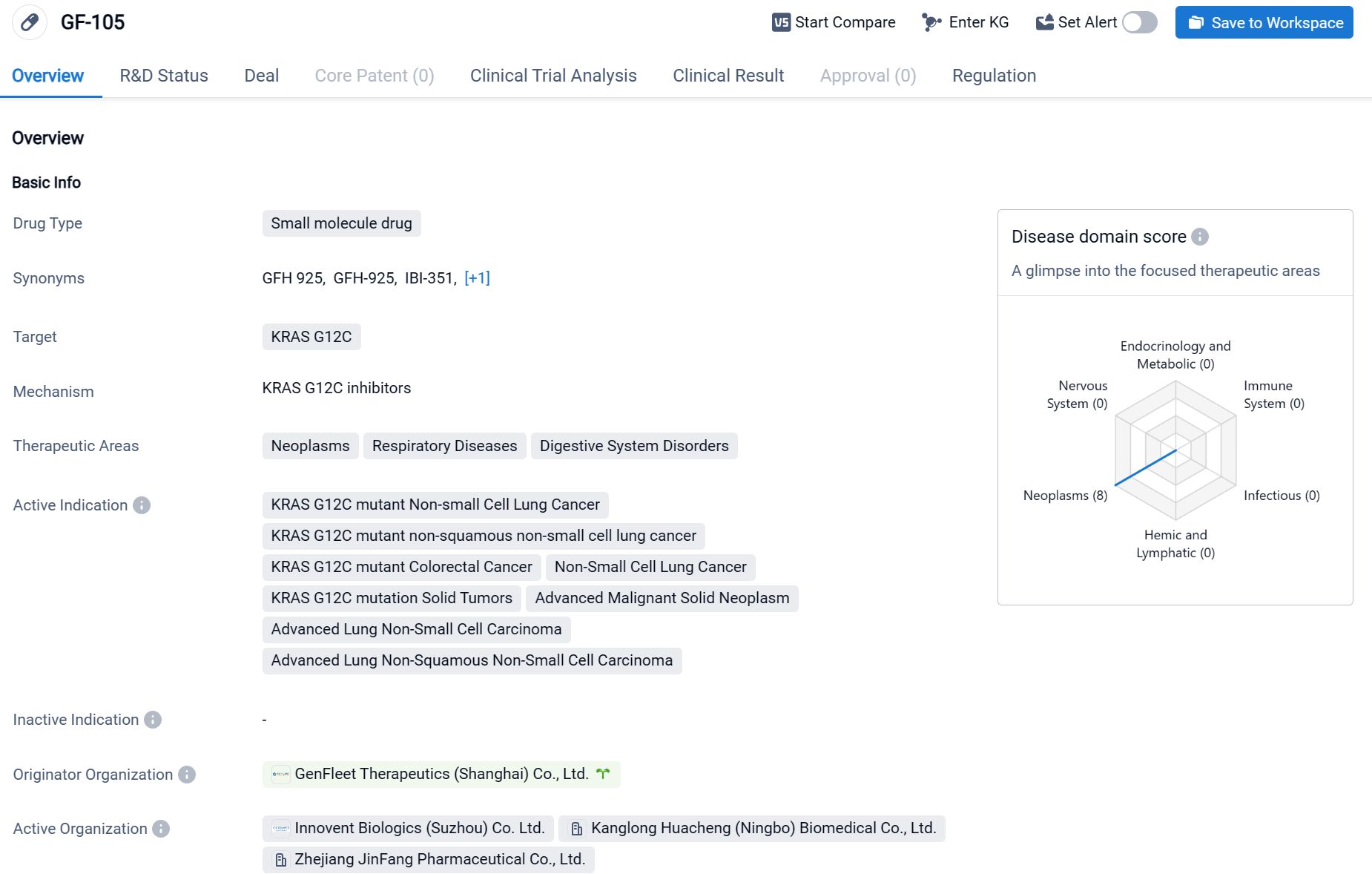
Recently, Innovent Biologics, Inc./GenFleet Therapeutics Co., Ltd. co-developed GF-105 marketing authorization application (NDA) was accepted by the CDE and included in the priority review process for patients with advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutation who have received at least one systemic therapy. GFH925 (IBI351) is the first KRAS G12C inhibitor in China to have its NDA submitted for acceptance into the priority review process. In September 2021, Innovent and Genfleet announced that they had entered into an exclusive global licensing agreement, under which Innovent, as the exclusive partner, has been granted the development and commercialization rights to GFH925 in China, including Mainland China, Hong Kong, Macau and Taiwan, and has global development and commercialization rights. commercialization rights, with an option for global development and commercialization rights.
About RBD7022
GF-105 is a small molecule drug developed by GenFleet Therapeutics (Shanghai) Co., Ltd. It is designed to target the KRAS G12C mutation, which is associated with various types of cancers. The active indications for GF-105 include KRAS G12C mutant Non-small Cell Lung Cancer, KRAS G12C mutant non-squamous non-small cell lung cancer, KRAS G12C mutant Colorectal Cancer, Non-Small Cell Lung Cancer, KRAS G12C mutation Solid Tumors, Advanced Malignant Solid Neoplasm, Advanced Lung Non-Small Cell Carcinoma, and Advanced Lung Non-Squamous Non-Small Cell Carcinoma. GF-105 has reached the highest phase of development, which is NDA/BLA (New Drug Application/Biologics License Application) globally. Click the image below to directly embark on the exploration journey with the RBD7022!
According to the data disclosed at the 2023 AACR conference: As of February 10, 2023, among the 67 evaluable NSCLC (non-small cell lung cancer) patients, the ORR (Objective Response Rate) was 61.2%, and the DCR (Disease Control Rate) was 92.5%; particularly, the 600mg BID (twice daily) dosage group (recommended Phase 2 Dose, RP2D) demonstrated superior efficacy, with an ORR of 66.7% (20 out of 30 evaluable patients), including a confirmed ORR (cORR) of 53.3% (16 out of 30), and a DCR of 96.7%.
Additionally, regarding CRC (colorectal cancer) indications, as per the data released at the 2023 ASCO conference: As of February 16, 2023, a total of 54 patients with advanced colorectal cancer were included in the analysis. In the 600mg BID dosage group, 42 patients had undergone at least one tumor assessment, with an ORR of 42.9% (18 out of 42), a cORR of 31.0% (13 out of 42), and a DCR of 88.1% (37 out of 42).
About GenFleet Therapeutics Co., Ltd.
GenFleet Therapeutics Co., Ltd. is a relatively young biomedicine organization that has made significant progress in its drug development efforts. With a strong focus on Neoplasms and a diverse range of therapeutic areas, the company is actively working towards developing innovative treatments for various diseases. The pipeline indicates a focus on early-stage development, with a significant number of drugs in the preclinical stage. However, further progress is needed to advance these drugs into later stages of development and ultimately gain regulatory approval.
Since its establishment in 2017, GenFleet has established over ten proprietary "globally innovative" large and small molecule projects, with multiple products entering global multi-center clinical trials in China, Europe, the United States, and Australia, of which more than five clinical studies have entered the Phase II application and development stage.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
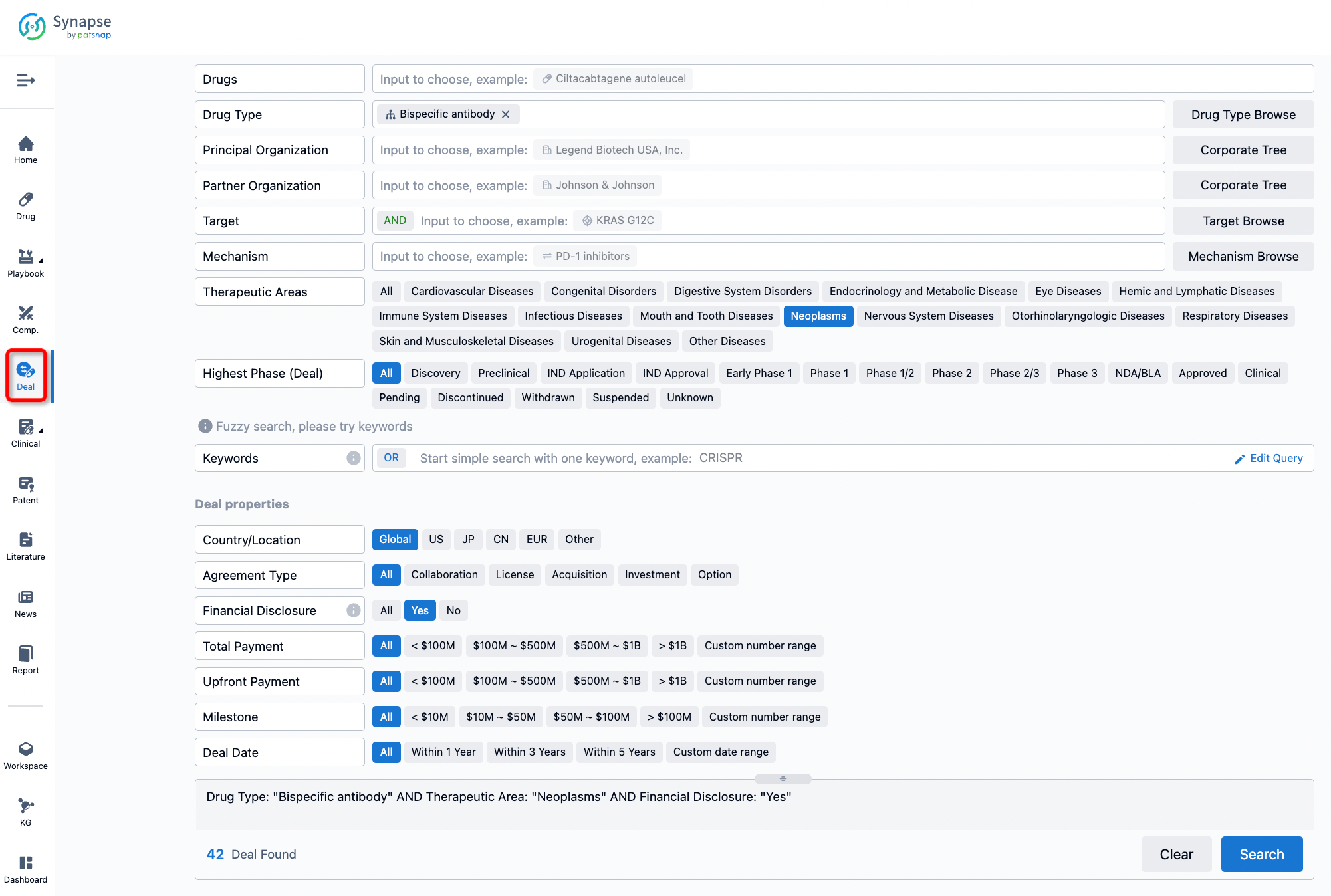
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
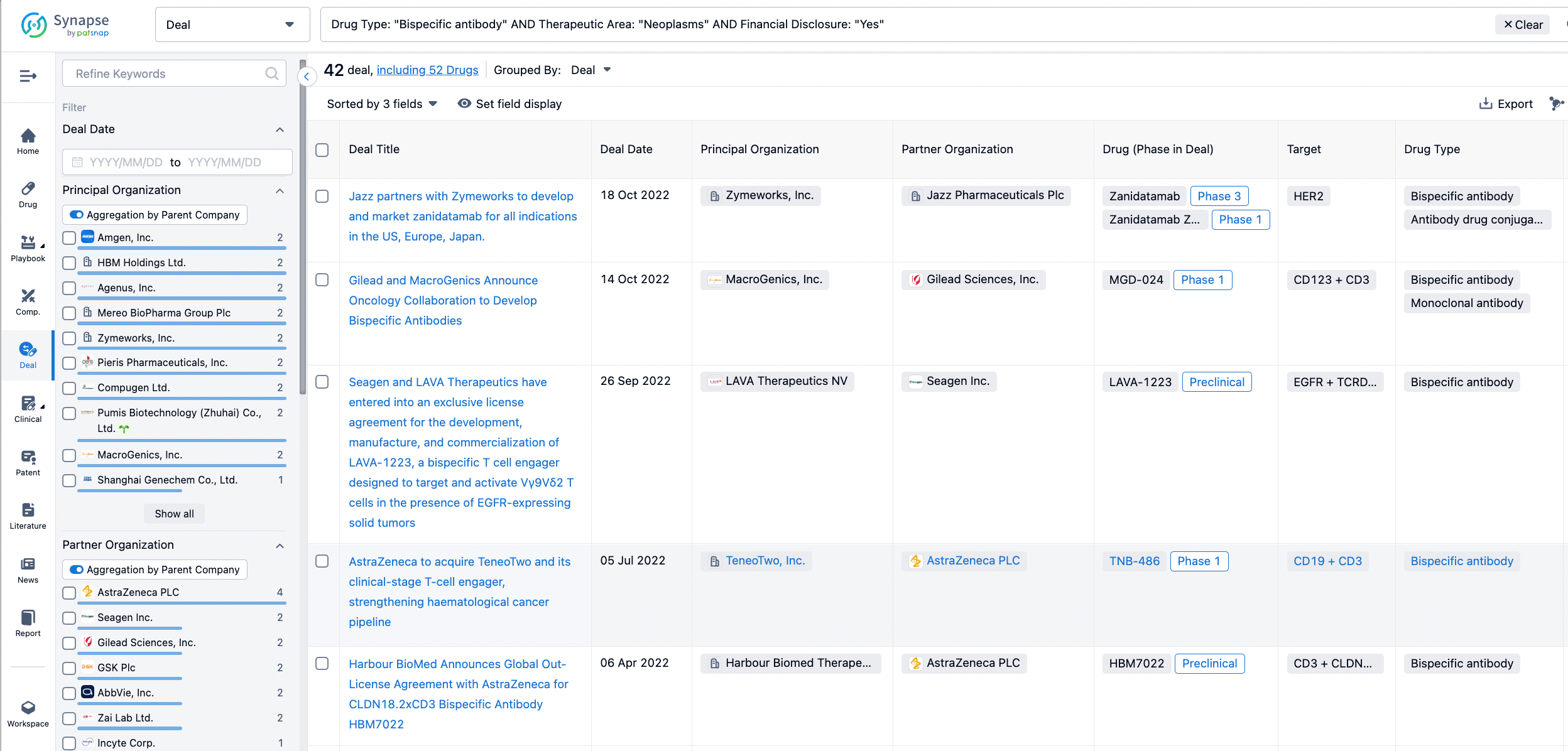
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
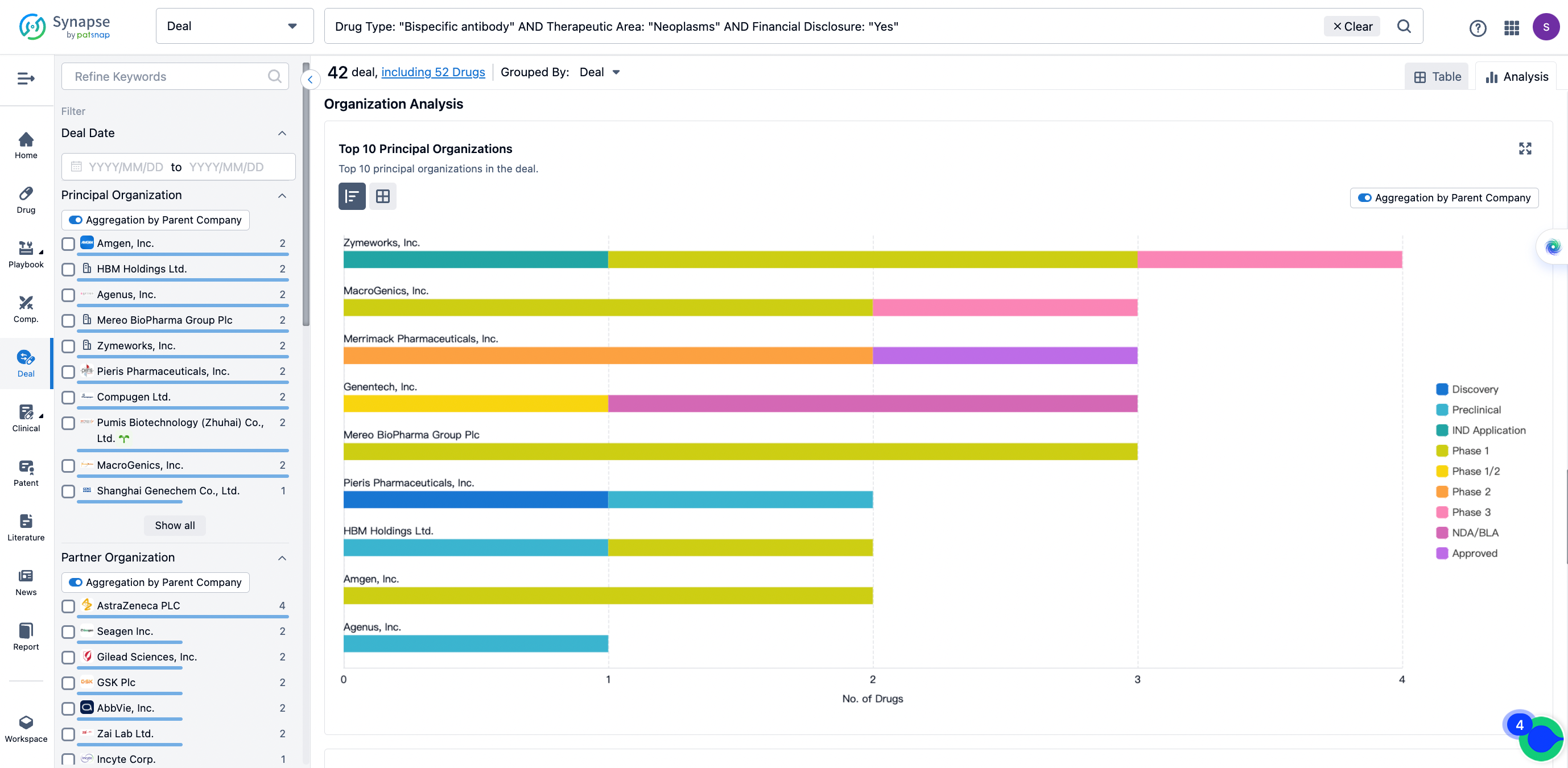
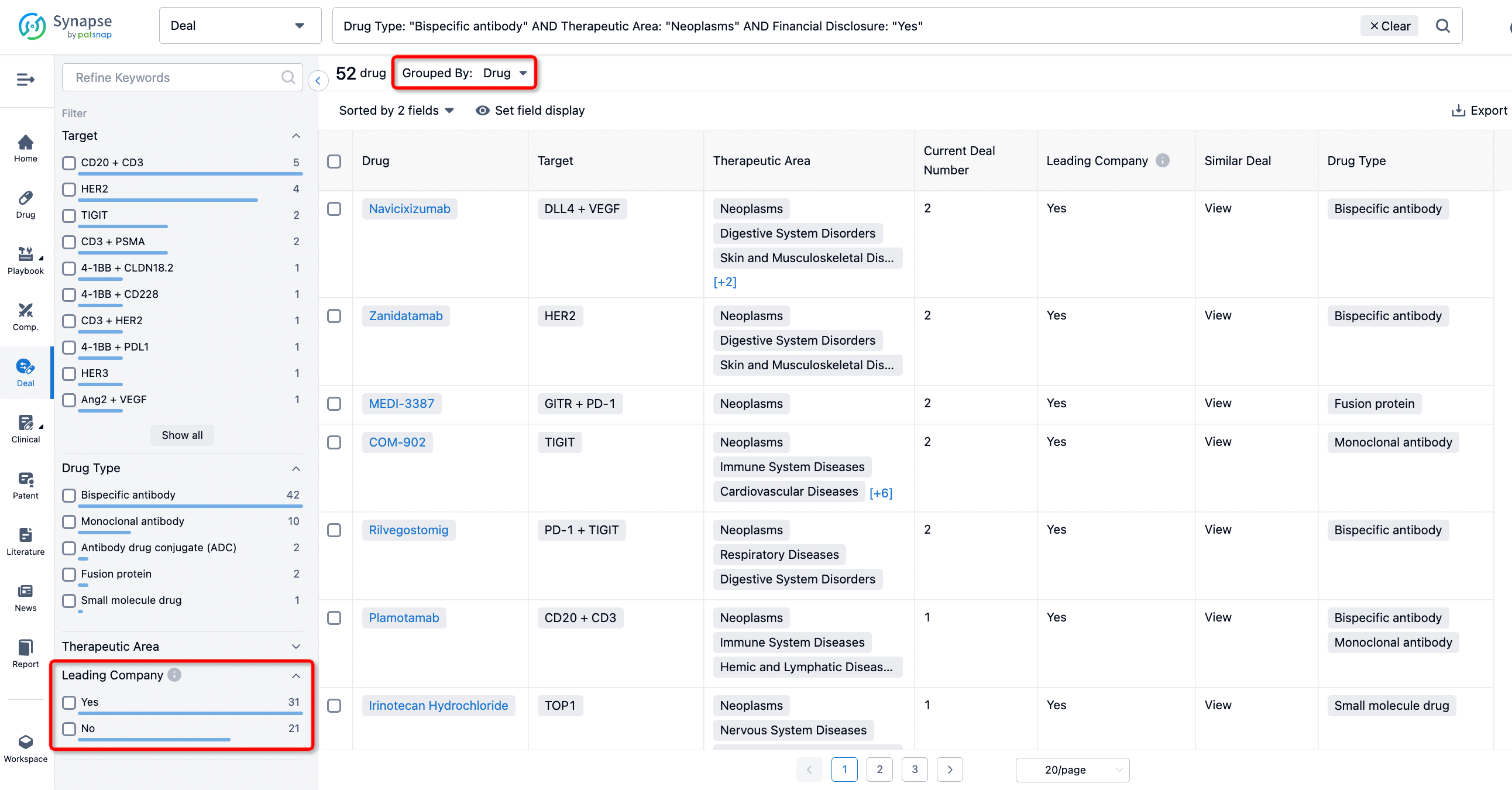
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
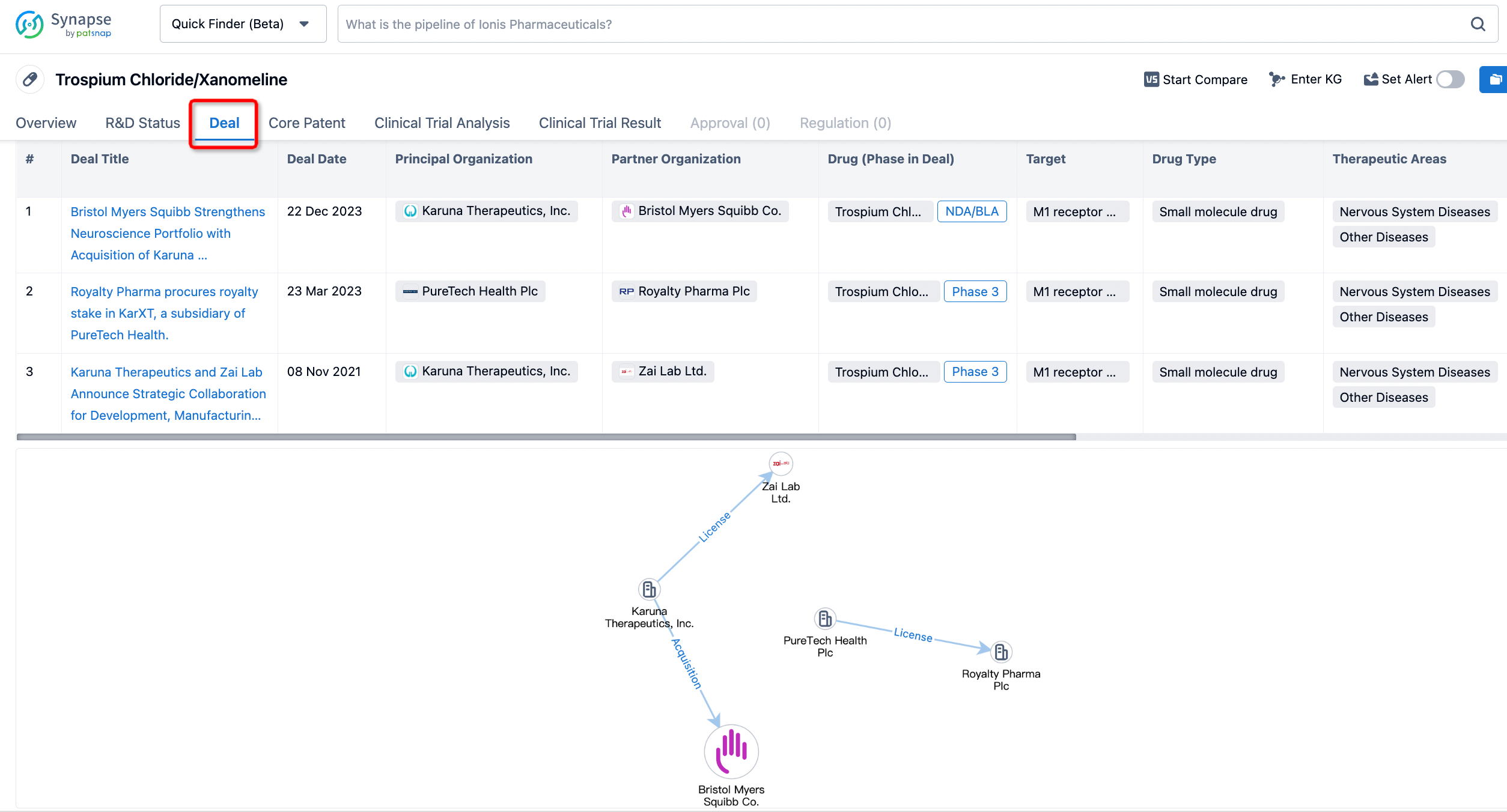
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
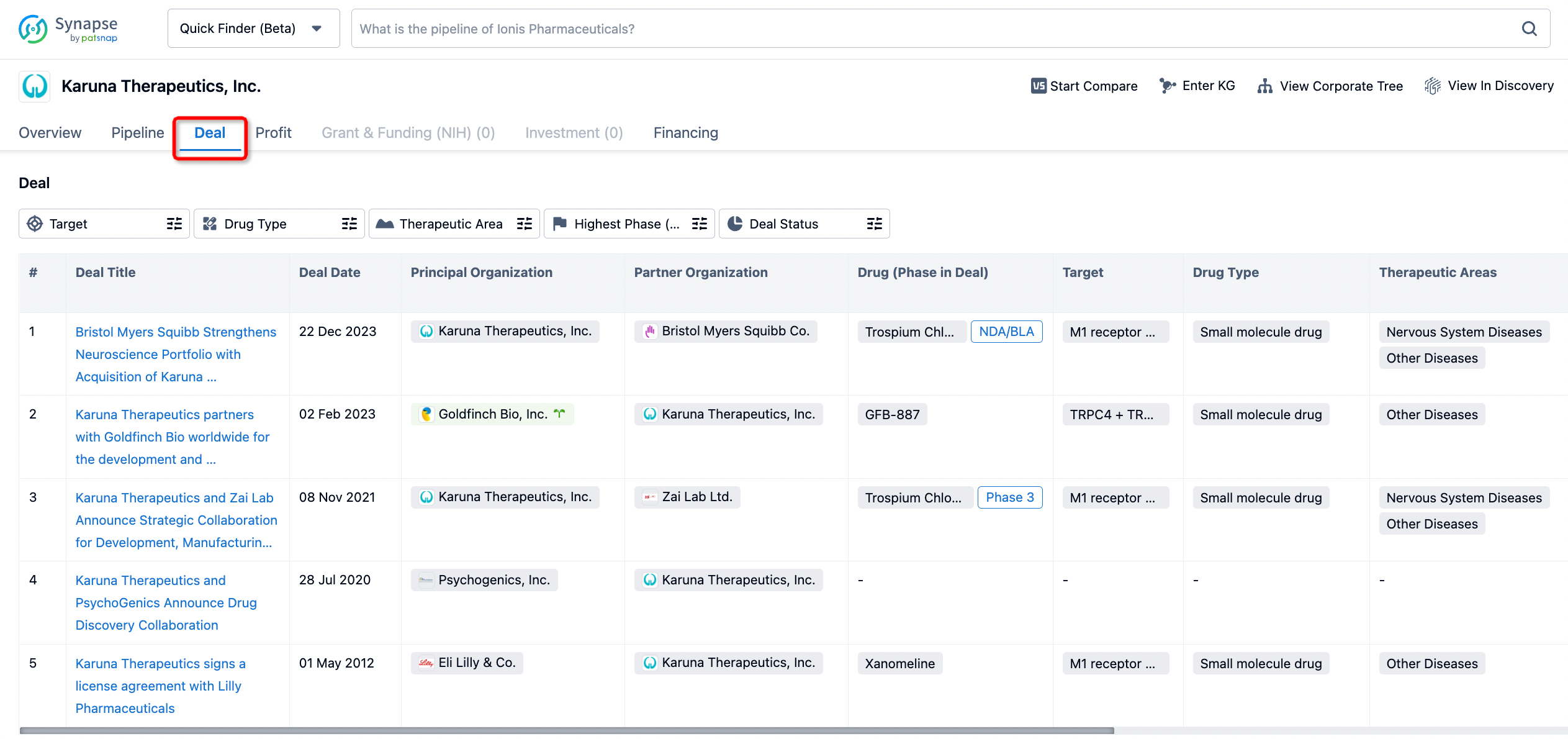
Click on the image below to embark on a brand new journey of drug discovery!