EU Grants Authorization for New Eylea™ 8 mg Formulation
The European Commission has provided commercial approval within the EU for the newly introduced Eylea™ 8 mg, a concentrated injectable solution with a dose of aflibercept 8 mg (114.3 mg/ml), destined for the medical management of two significant conditions of the retina: neovascular age-related macular degeneration and vision loss connected to diabetic macular edema.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Eylea, at the 8 mg dosage, has been sanctioned for usage at prolonged intervals of up to a quarter of a year after a trio of initial doses every month. For those exhibiting stable visual acuity, the possibility of extending the interval to a quintet of months may be explored. This particular dosage of Eylea stands as the sole therapeutic option within the European Union granted sanction for such extensive dosing intervals, reaching up to 5 months, for the conditions of nAMD and DME.
Dr. Michael Devoy, presiding as the Chief Medical Officer for the Pharmaceuticals Division of Bayer, expressed significance in the EU's sanctioning of Eylea 8 mg. "This authorization within the EU serves as a notable landmark in the ongoing effort to alleviate the burden endured by patients with retinal ailments," he stated. "Eylea 2 mg established the foundational treatment regimen and now, with Eylea 8 mg, patients may experience benefits at reduced injection frequency, whilst maintaining comparable results in efficacy and safety."
The endorsement within the EU leverages encouraging outcomes derived from two pivotal studies: the PULSAR trial pertaining to nAMD and the PHOTON study examining DME. Each of these clinical investigations achieved its primary objective, demonstrating that changes in the best-corrected visual acuity with aflibercept 8 mg, using dosing intervals of either 12 or 16 weeks, were non-inferior to that accomplished with aflibercept 2 mg (Eylea 40 mg/ml) on a fixed 8-week dosing cycle at the 48-week mark. Notably, the safety profile associated with aflibercept 8 mg paralleled the recognized safety paradigm of Eylea (aflibercept 2 mg).
The American FDA endorsed aflibercept 8 mg, marketed as Eylea HD, in the month of August 2023. In pursuit of broader global access, Bayer has filed additional market regulatory applications for aflibercept 8 mg. Spearheading the collaborative development of aflibercept 8 mg are Bayer and Regeneron. While Regeneron exclusively retains the rights for both Eylea (aflibercept 2 mg) and Eylea HD in the U.S., Bayer has been granted exclusive rights for marketing these products outside of the U.S., with both companies sharing profits from the sales of Eylea variants equally.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
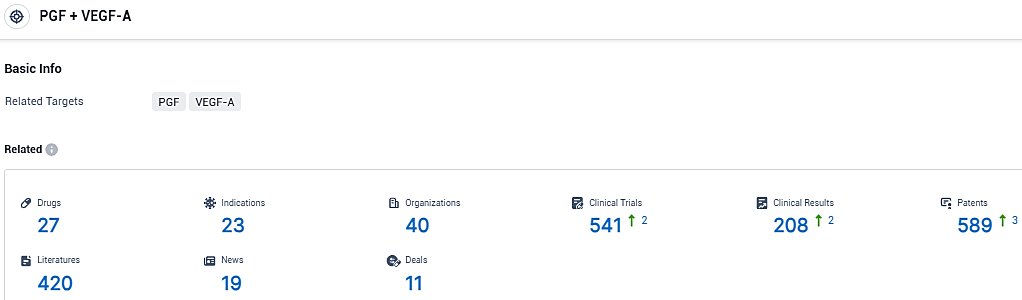
According to the data provided by the Synapse Database, As of January 12, 2024, there are 27 investigational drugs for the PGF and VEGF-A tagets, including 23 indications, 40 R&D institutions involved, with related clinical trials reaching 541, and as many as 589 patents.
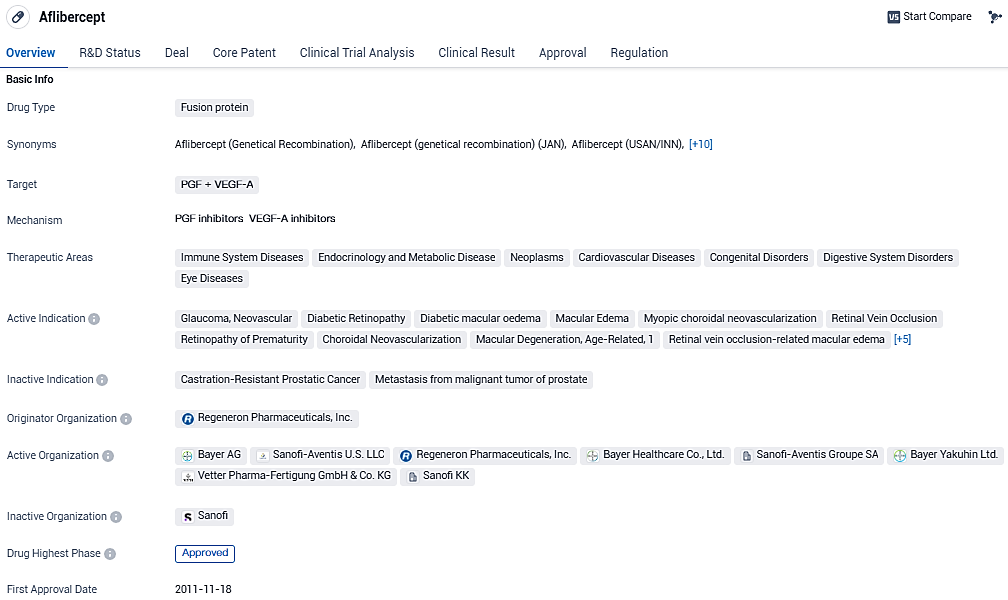
Aflibercept targets PGF and VEGF-A and has been approved for various therapeutic areas, including immune system diseases, endocrinology and metabolic disease, neoplasms, cardiovascular diseases, congenital disorders, digestive system disorders, and eye diseases. Its approval in the United States and China, along with its breakthrough therapy designation and orphan drug status, highlight its potential as a versatile and effective treatment option for multiple indications.