Exploring the Latest PCSK9 siRNA Deal by Suzhou Ribo Life Science: A Guide to Rapidly Accessing Transaction Insights
Recently, Suzhou Ribo Life Science announced the signing of a licensing agreement with Qilu Pharmaceutical. Under this agreement, the rights for the development, production, and commercialization of the PCSK9-targeting antisense oligonucleotide drug, RBD7022 (SR043), have been granted to Qilu Pharmaceutical for the Greater China region, which includes Mainland China, Hong Kong, and Macau. Suzhou Ribo Life Science will receive an upfront and milestone payments totaling more than 700 million RMB, in addition to royalties that could reach double-digit percentages.
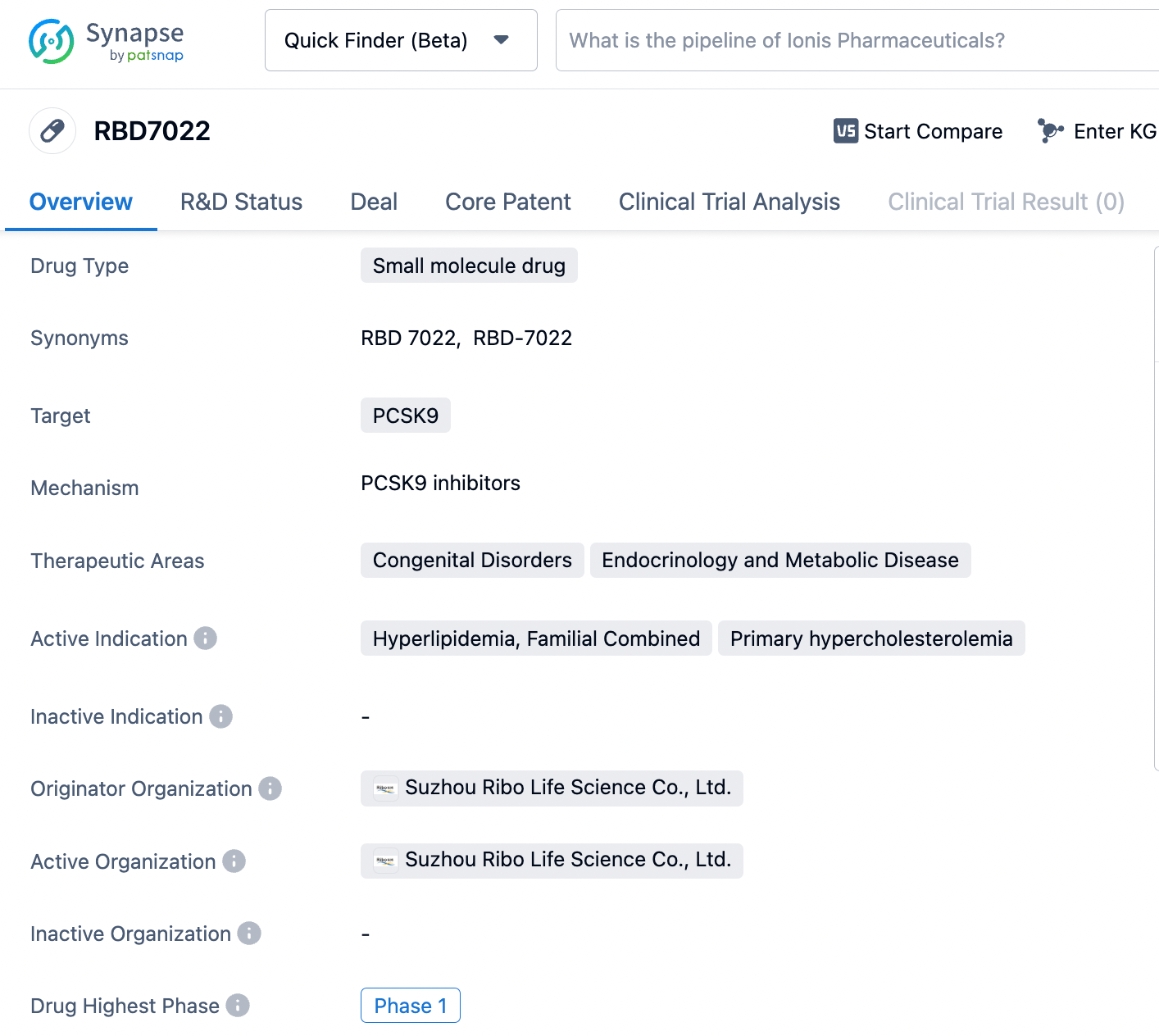
About RBD7022
RBD7022 is a GalNAc-conjugated small interfering RNA (siRNA) drug developed by Suzhou Ribo Life Science based on its proprietary RIBO-GalSTAR® liver-targeting delivery technology for the treatment of hyperlipidemia. The drug functions by inhibiting the expression of PCSK9 protein, reducing lysosomal degradation of low-density lipoprotein receptors (LDL-R), and consequently lowering blood levels of LDL cholesterol (LDL-C). RBD7022 is intended for use in patients with familial hypercholesterolemia and those with atherosclerotic cardiovascular disease (ASCVD) whose LDL-C levels remain poorly controlled despite statin therapy. Currently, RBD7022 is in Phase I clinical development. Click the image below to directly embark on the exploration journey with the RBD7022!
The Phase I study is a randomized, single-blind, placebo-controlled, single-center trial with single ascending doses and multiple ascending doses designed to evaluate the safety, tolerability, pharmacokinetics, and preliminary pharmacodynamic characteristics of subcutaneously injected RBD7022 in subjects with normal or elevated levels of LDL cholesterol. The trial is actively recruiting participants.
Preclinical trial data indicate that RBD7022 has favorable safety characteristics and a potent lipid-lowering effect. The drug's lipid-lowering action is characterized by stable levels of lipid reduction and durable efficacy, with a single administration sustaining efficacy for several months and achieving an inhibition of LDL-C by over 50%.
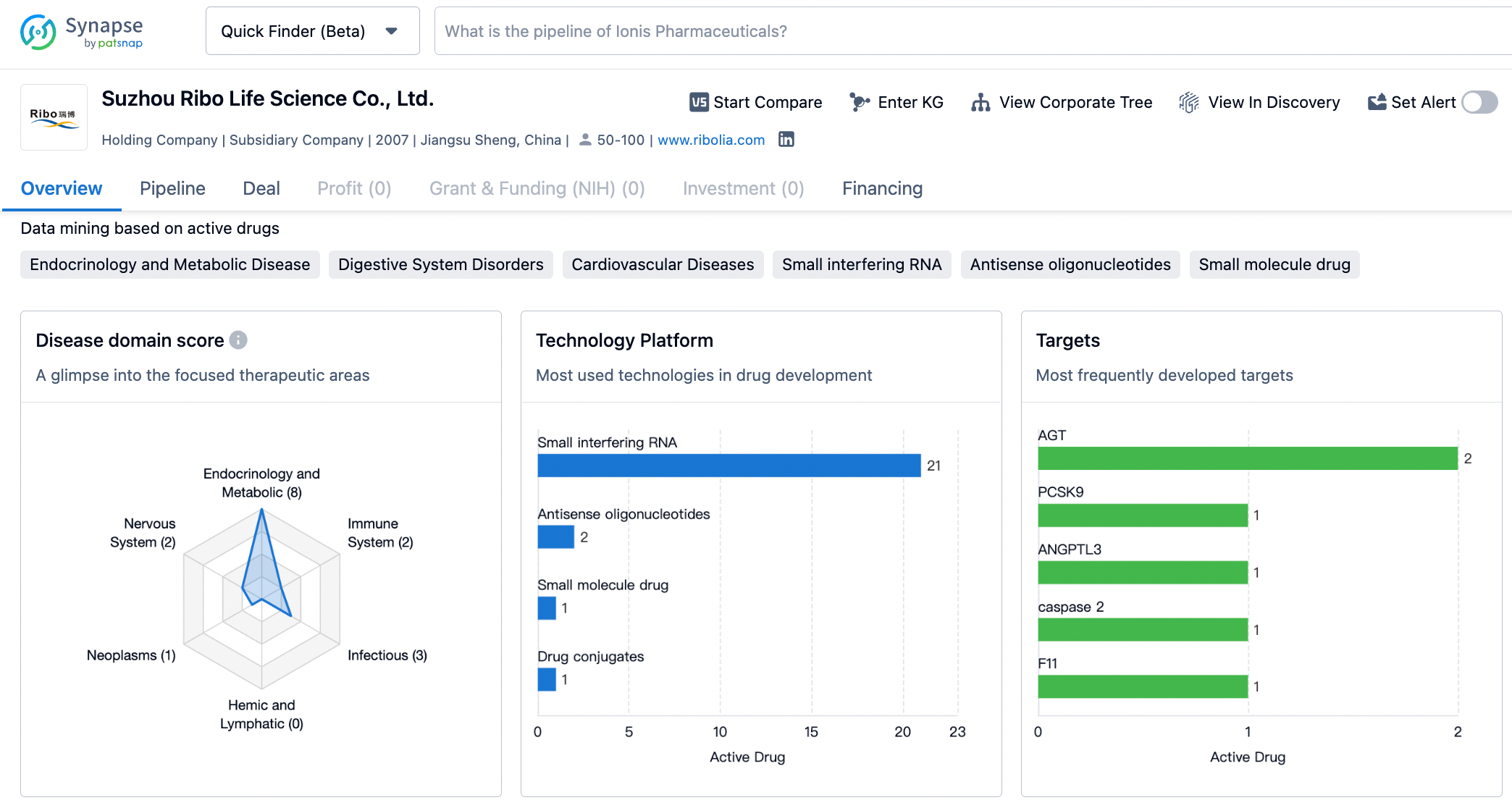
About Suzhou Ribo Life Science
Established in 2007, Suzhou Ribo Life Science Co., Ltd. (Ribo) has become a clinical stage company that is dedicated to the advancement of innovative RNAi technologies and oligonucleotide therapeutics. Its efforts are focused on addressing unmet clinical needs worldwide with the use of tools and RNA therapeutics developed in China. Driven by a vision to have China's oligonucleotide therapeutics benefit the global community, the company is consistently exploring new avenues for growth and contribution to the field.
Leveraging state-of-the-art RNAi technologies, Ribo has created an oligonucleotide therapeutic platform that encompasses a comprehensive range of technologies. This platform is capable of supporting the entire life cycle of oligonucleotide therapeutics, spanning from early research and development through to full commercialization.
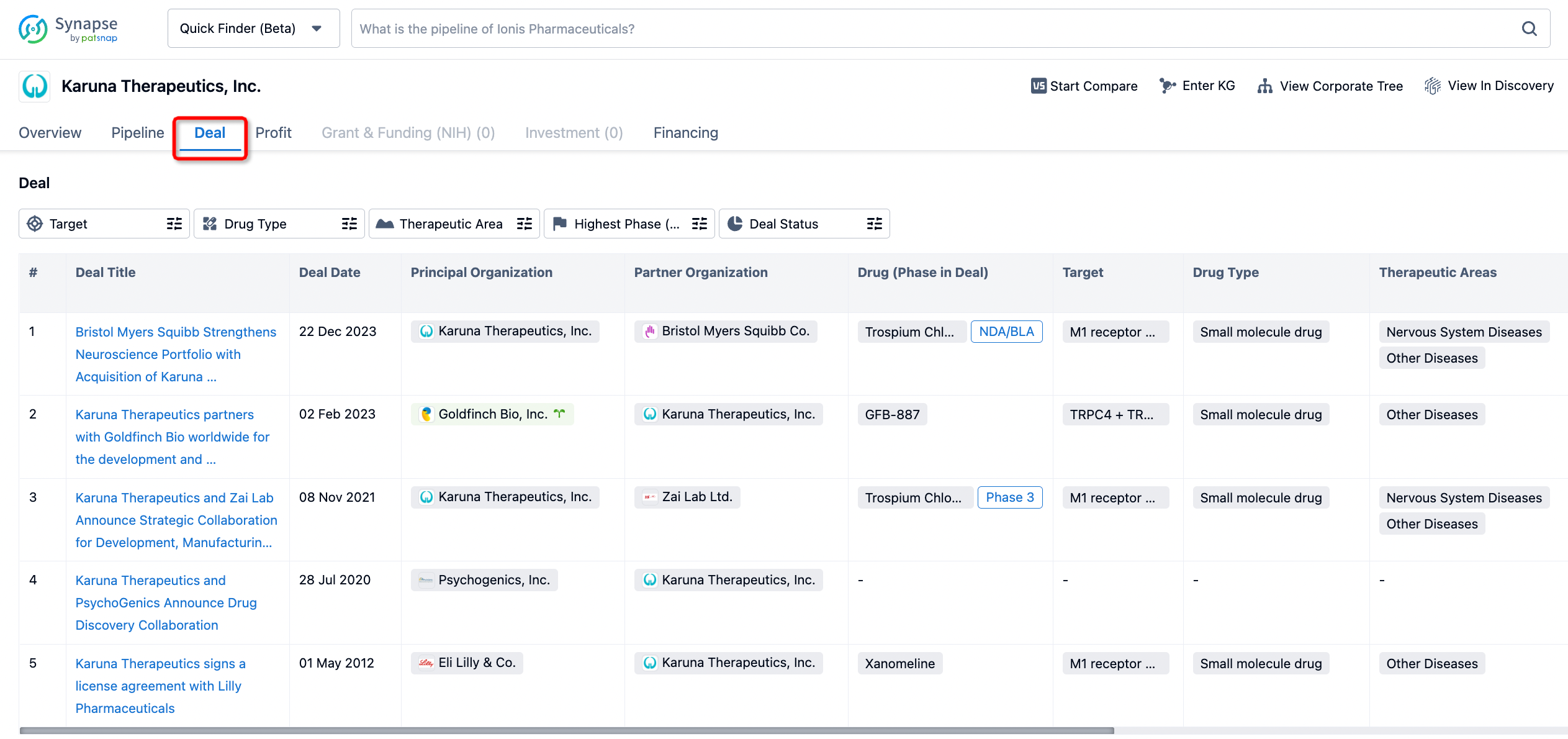
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
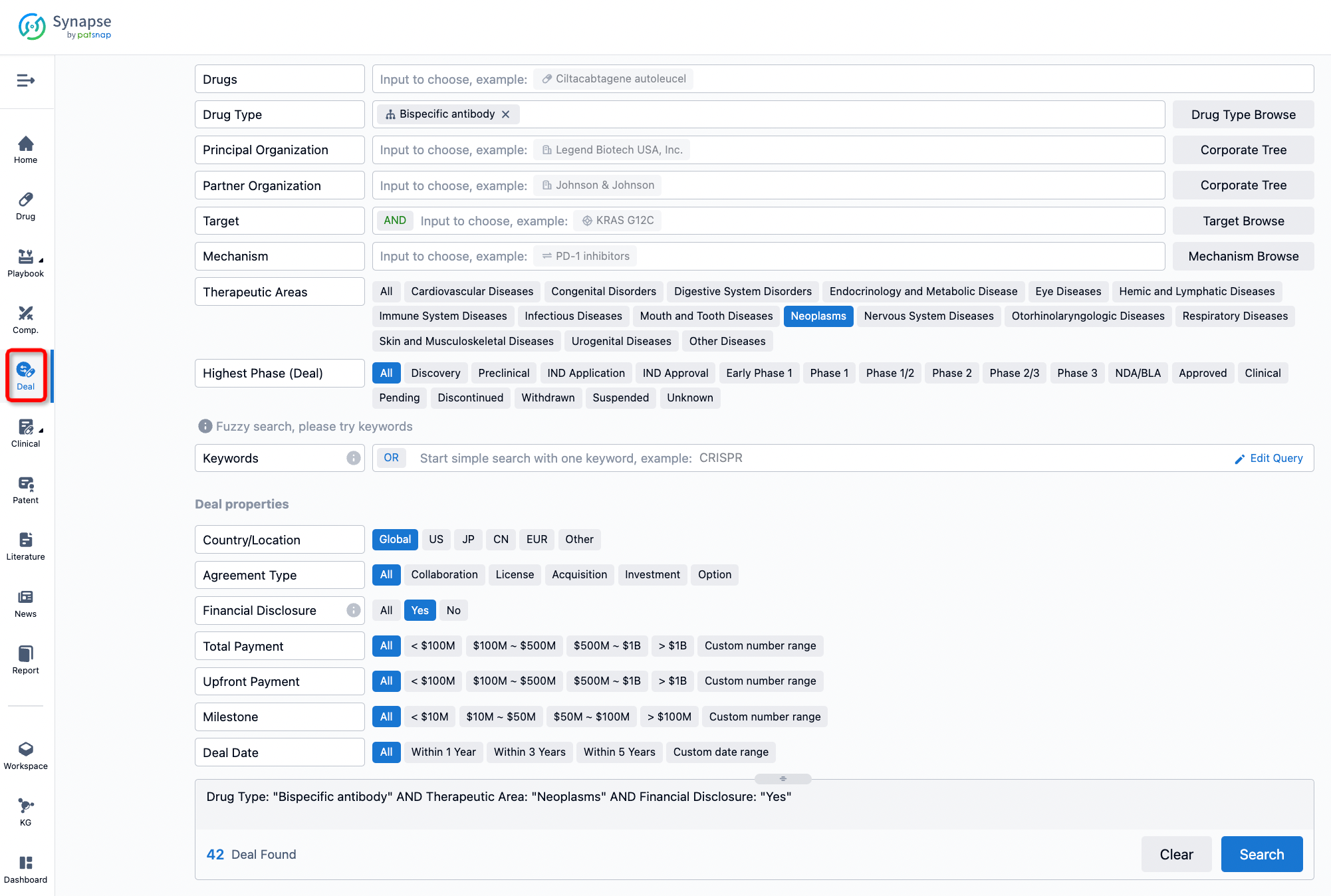
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
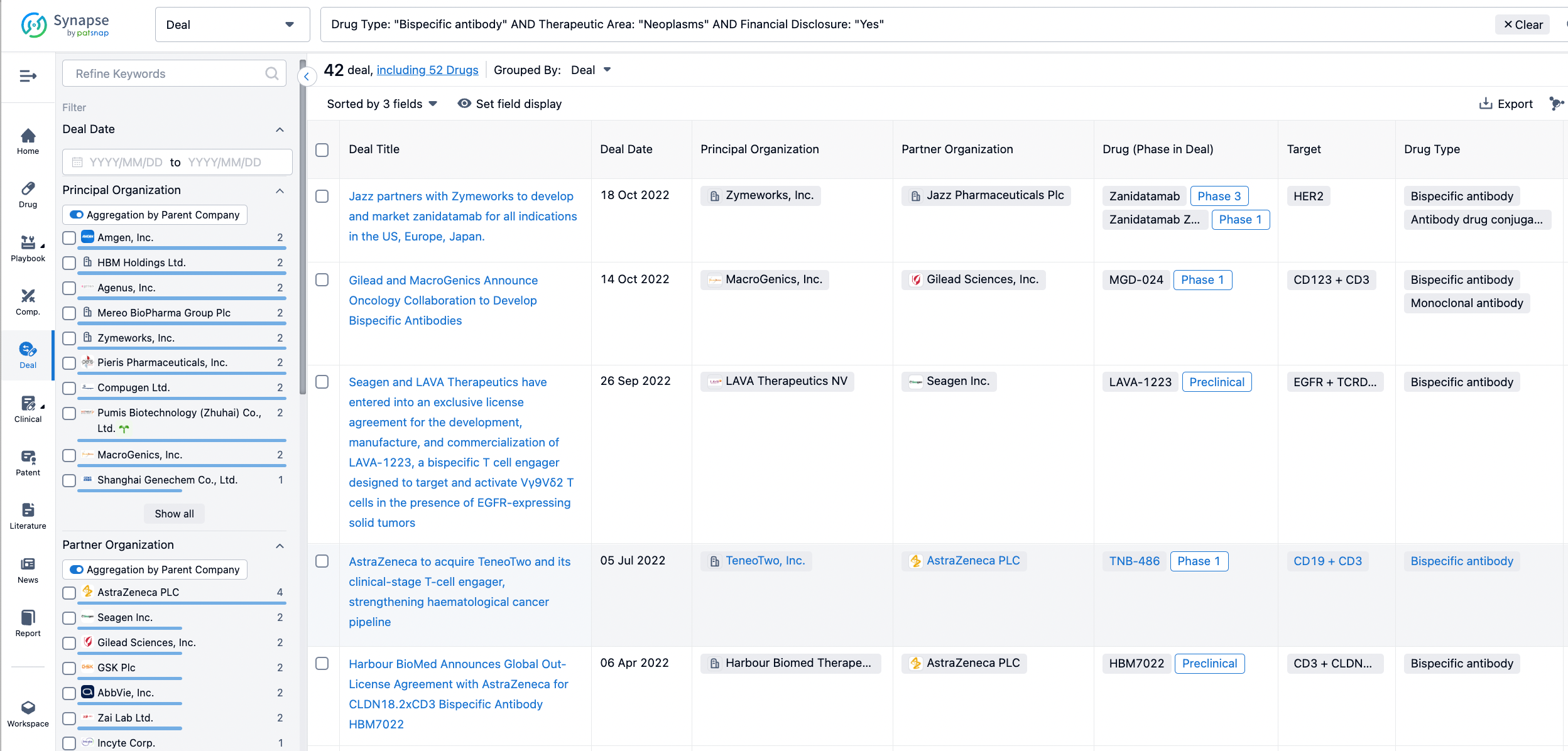
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
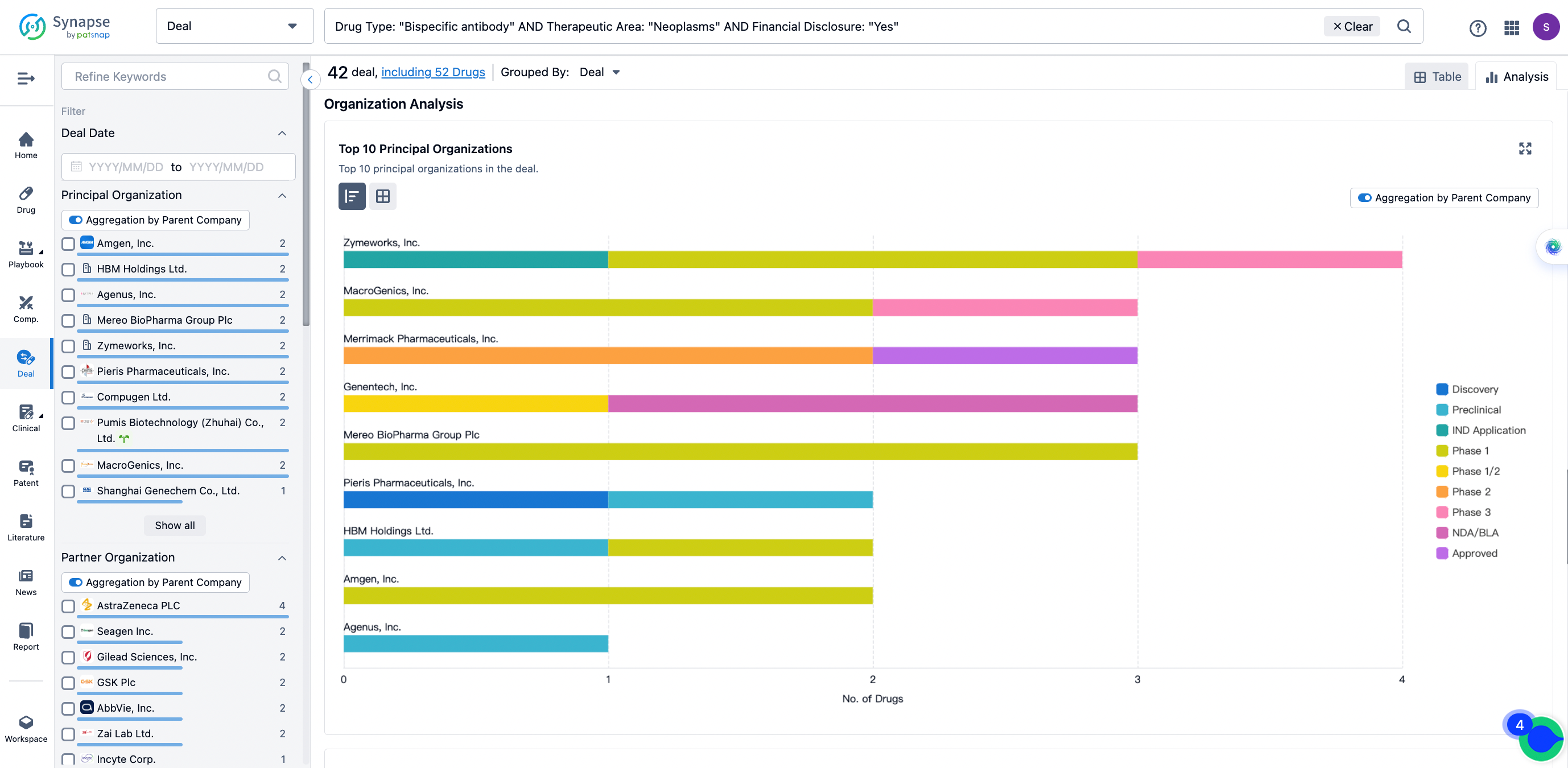
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
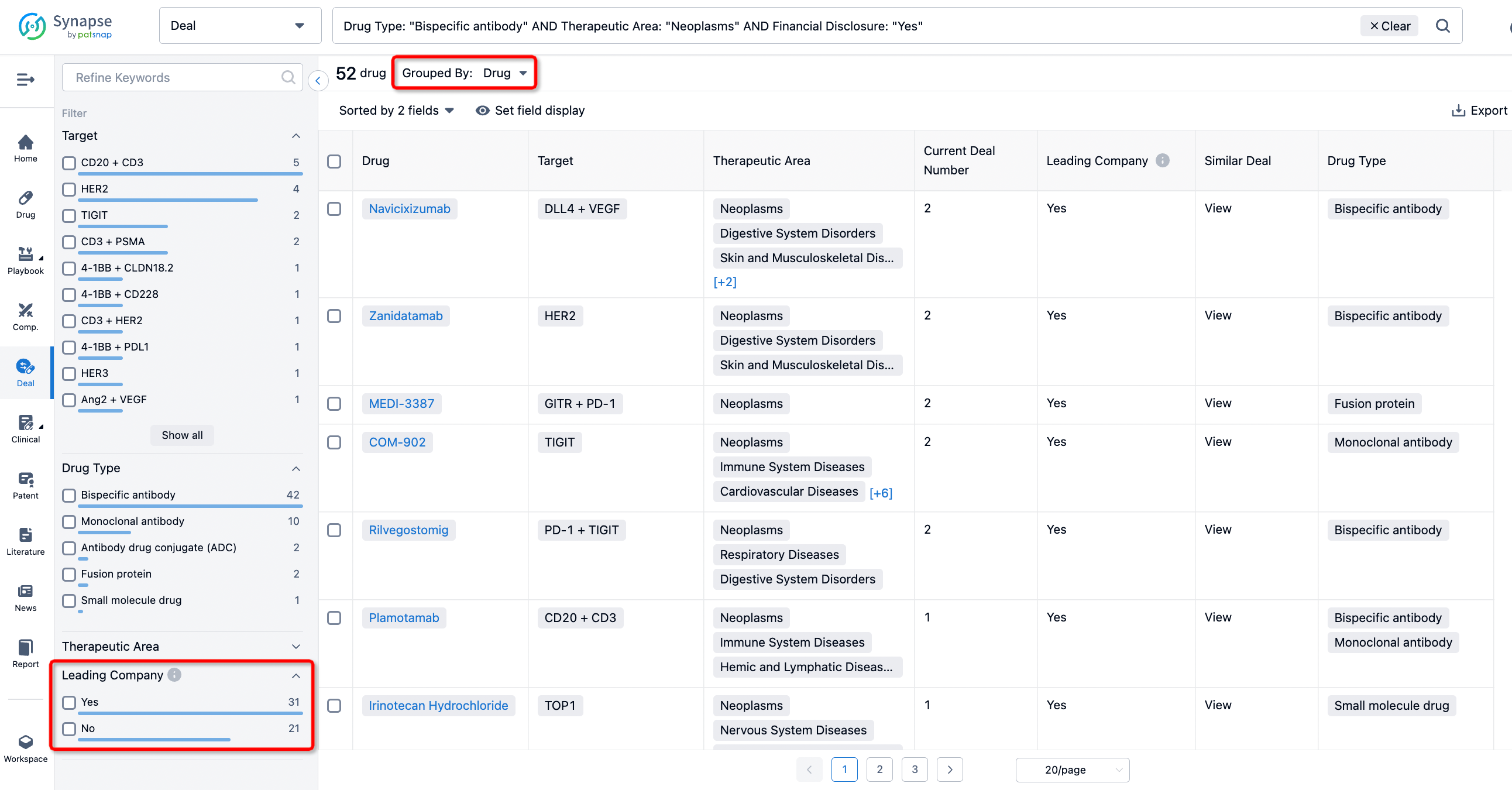
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
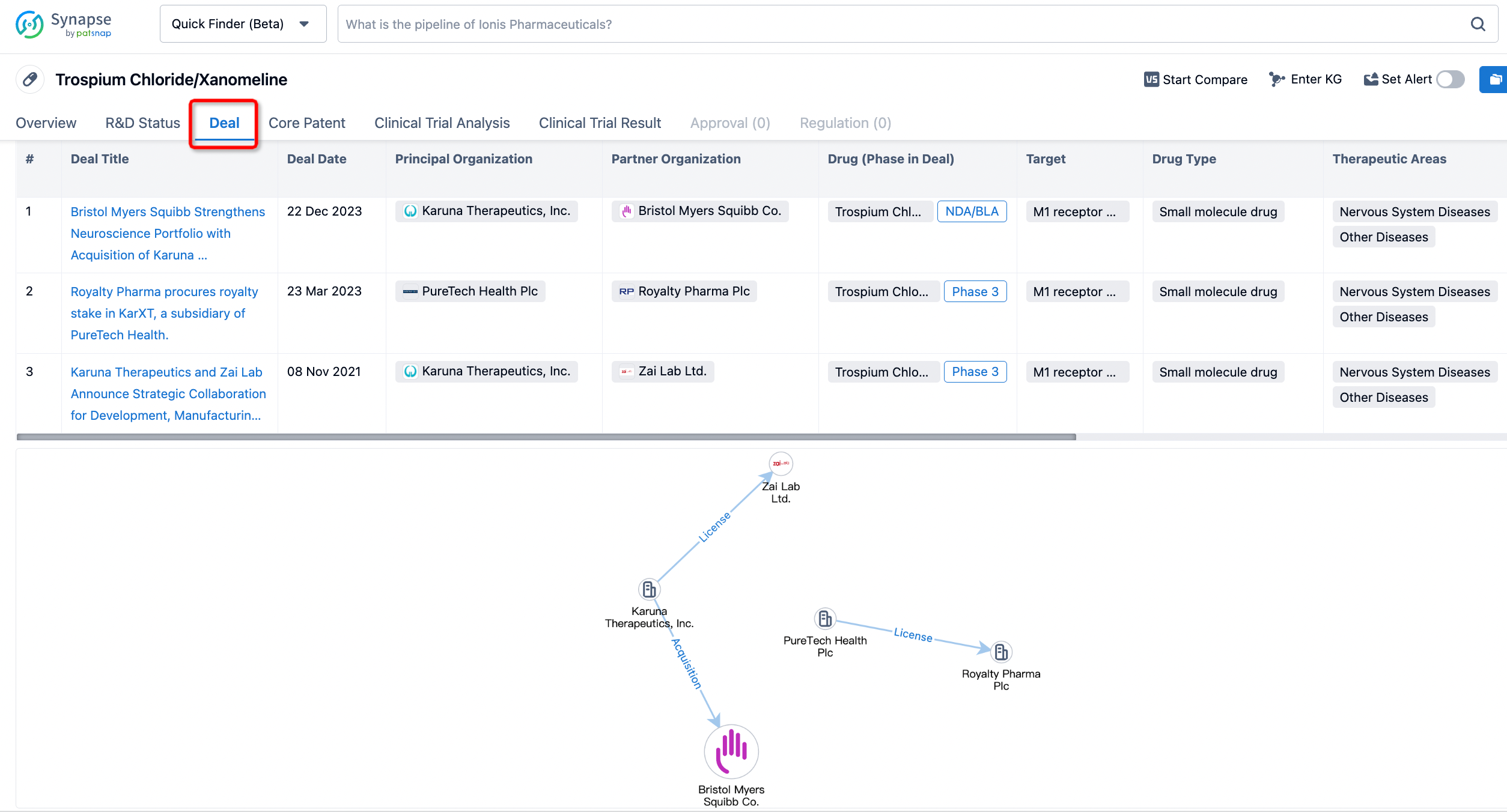
Click on the image below to embark on a brand new journey of drug discovery!