Exploring the Latest RDC Deal by RayzeBio: A Guide to Rapidly Accessing Transaction Insights
On December 26, 2023, Bristol Myers Squibb (BMS) and RayzeBio jointly announced that they have reached a definitive merger agreement whereby Bristol Myers Squibb will acquire RayzeBio for $62.50 per share in cash, valuing the total equity at approximately $4.1 billion, which amounts to about $3.6 billion after deducting an estimated $500 million in cash on RayzeBio's balance sheet. The transaction is expected to be completed in the first half of 2024. RayzeBio holds a leading and innovative position in the field of targeted radiotherapy based on actinium. The company has disclosed three products, with RYZ101 being the most advanced and anticipated.
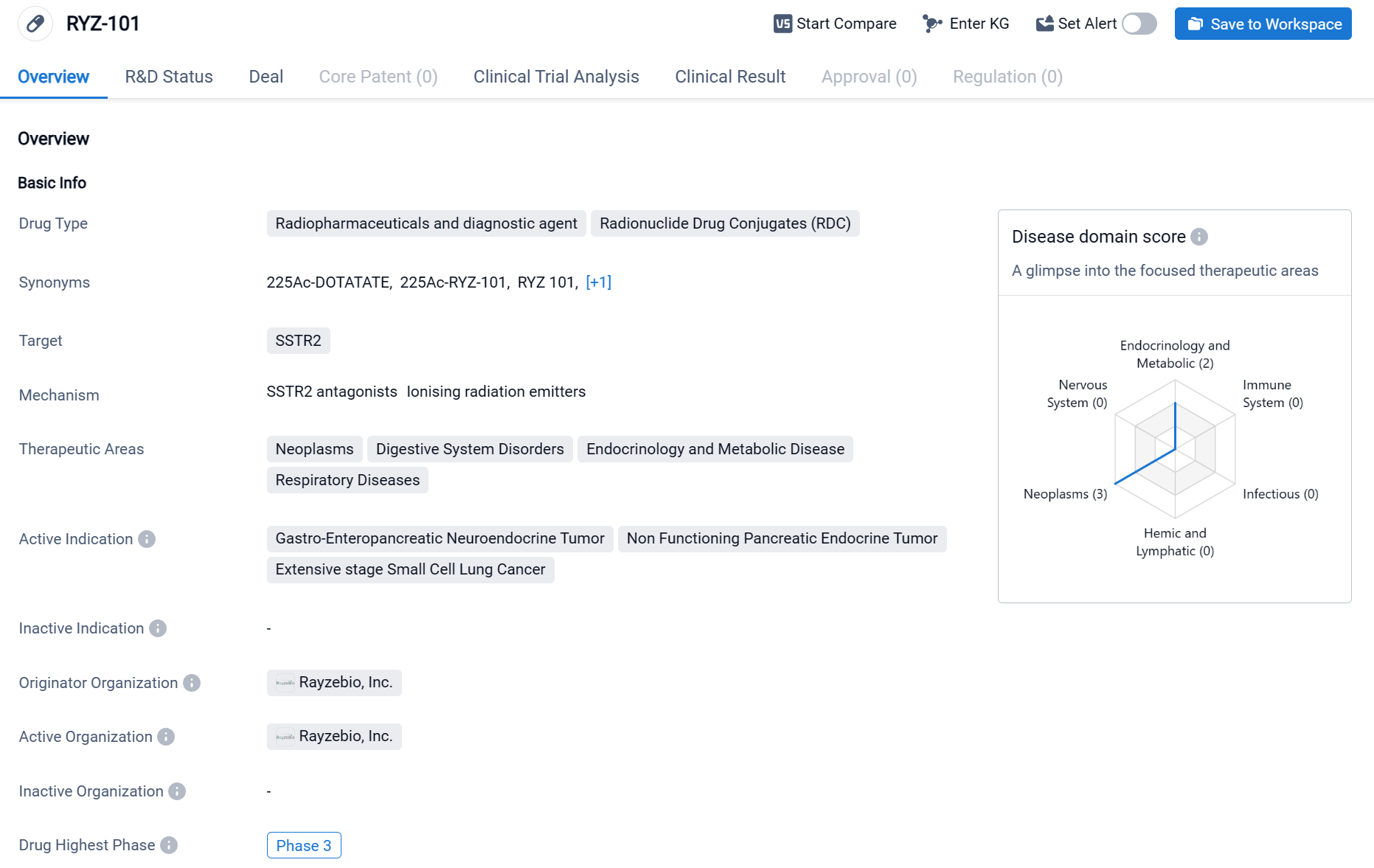
About RYZ-101
RYZ-101 is a radiopharmaceutical and diagnostic agent that falls under the category of Radionuclide Drug Conjugates (RDC). It primarily targets SSTR2, a receptor commonly found in various diseases. The drug has shown potential therapeutic applications in neoplasms, digestive system disorders, endocrinology and metabolic diseases, as well as respiratory diseases. Specifically, RYZ-101 has demonstrated efficacy in treating Gastro-Enteropancreatic Neuroendocrine Tumors (GEP-NETs), Non-Functioning Pancreatic Endocrine Tumors, and Extensive Stage Small Cell Lung Cancer. Click the image below to directly embark on the exploration journey with the RYZ-101!
Currently, RYZ101 is in Phase 3 clinical trials for patients with SSTR-positive GEP-NETs, and clinical trials for the treatment of extensive-stage small cell lung cancer (ES-SCLC) have entered Phase 1. If the Phase 3 clinical trials of RYZ101 are successful, it has the potential to become the world's first actinium-225 radiopharmaceutical.
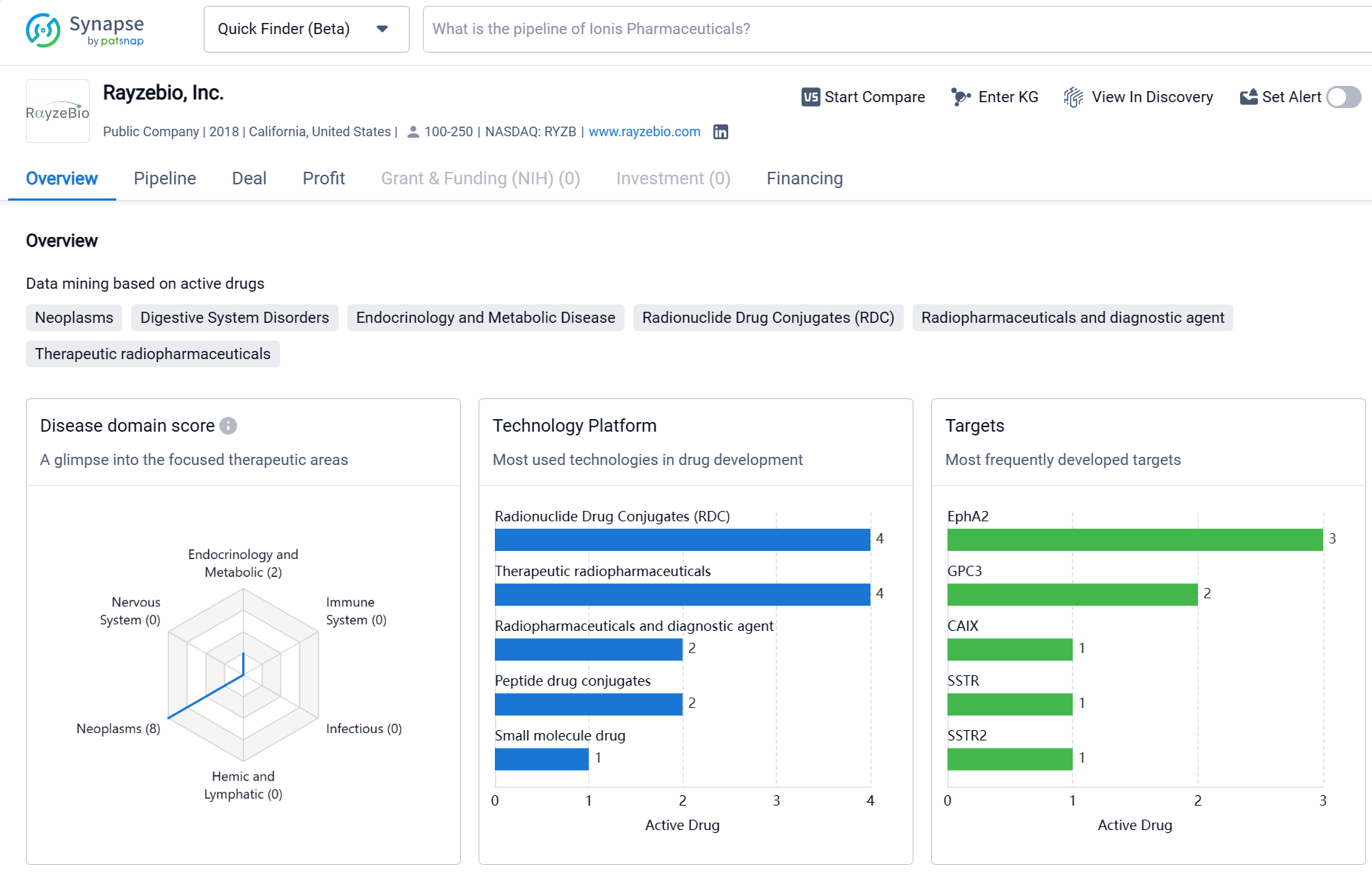
About Rayzebio
Rayzebio, Inc. is a biomedicine organization founded in 2018 and based in California, United States. The company has a strong focus on developing therapeutics for Neoplasms, with the highest drug count in this therapeutic area. It is also actively involved in developing treatments for Digestive System Disorders and Urogenital Diseases. The most frequently developed targets by Rayzebio, Inc. include EphA2 and GPC3. The company has a pipeline of drugs at various stages of development, with a significant number of drugs in the Preclinical phase. It has also progressed to Phase 2 and Phase 3 for some of its candidates. However, it has not yet received regulatory approval for any of its drugs. This information provides an objective overview of Rayzebio, Inc.'s activities in the pharmaceutical industry.
How to get the latest progress on drug deals?
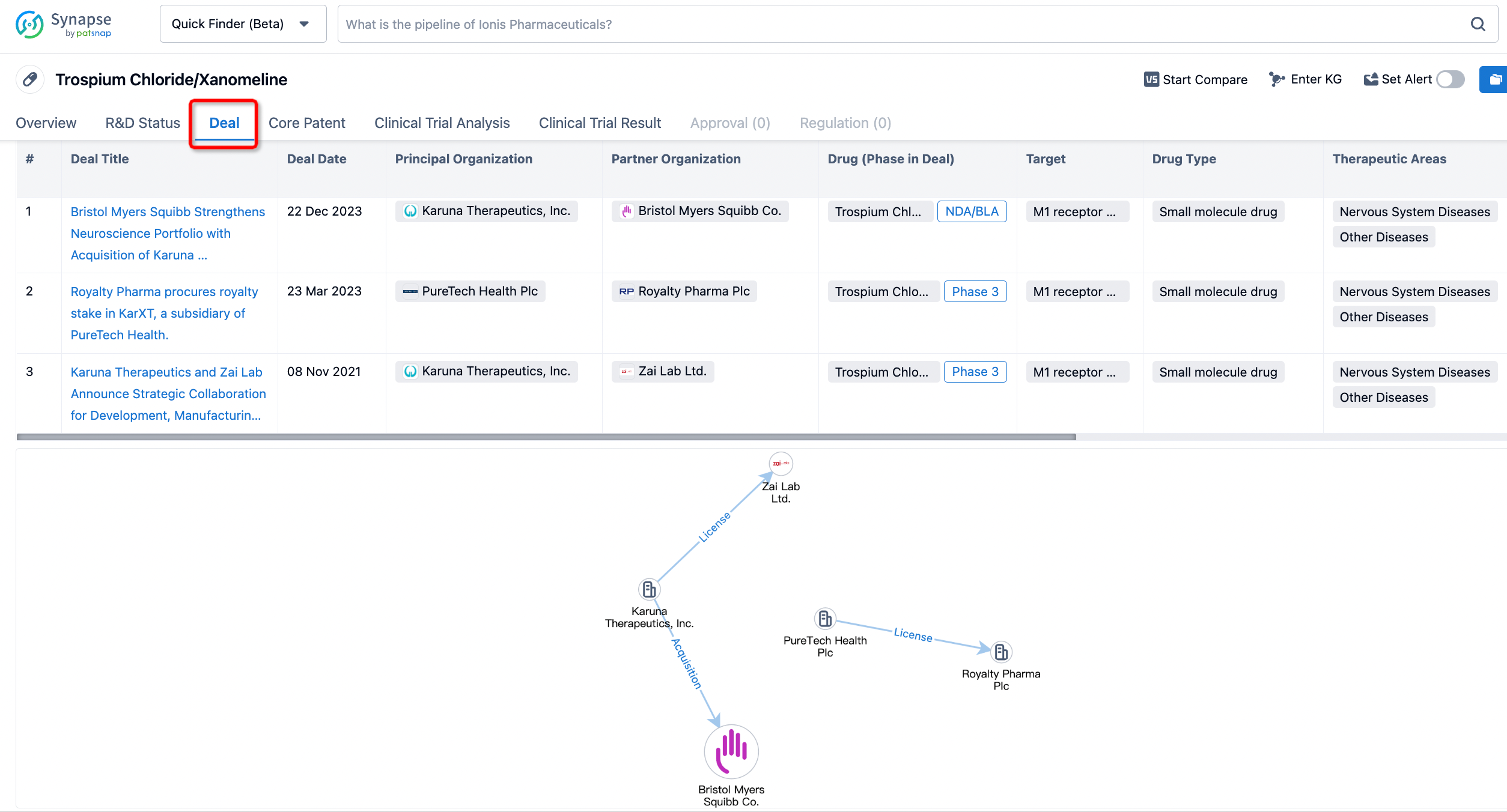
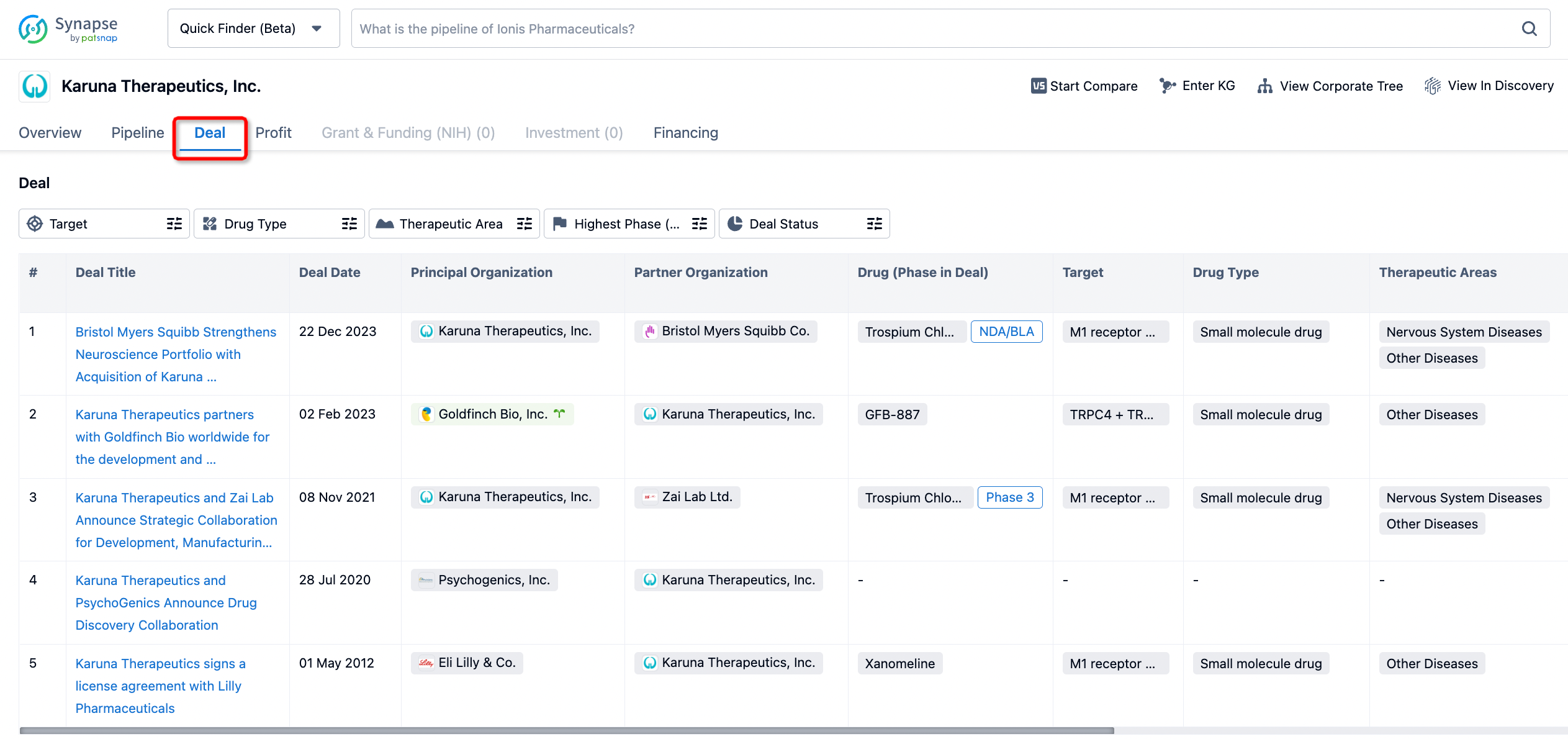
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
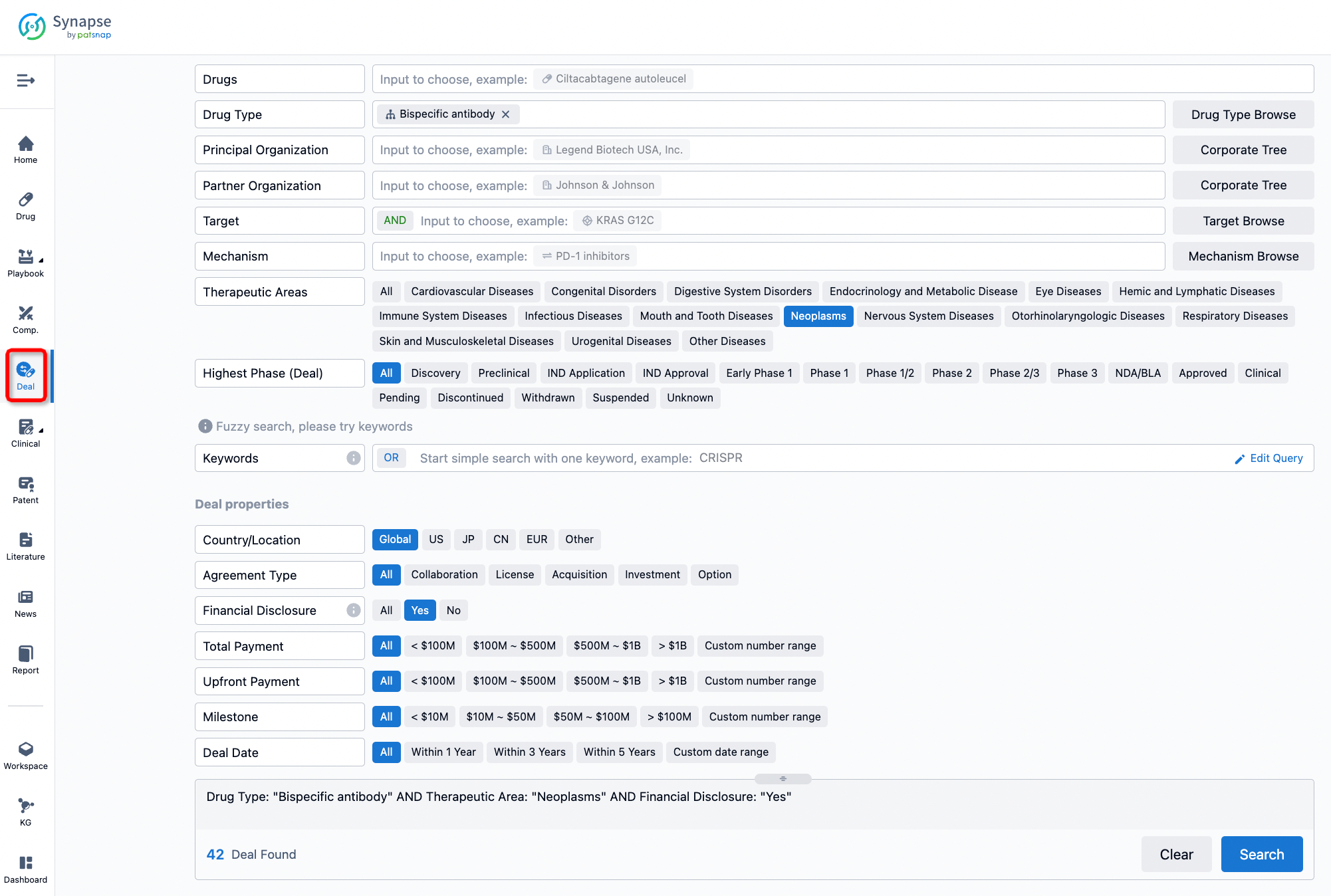
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
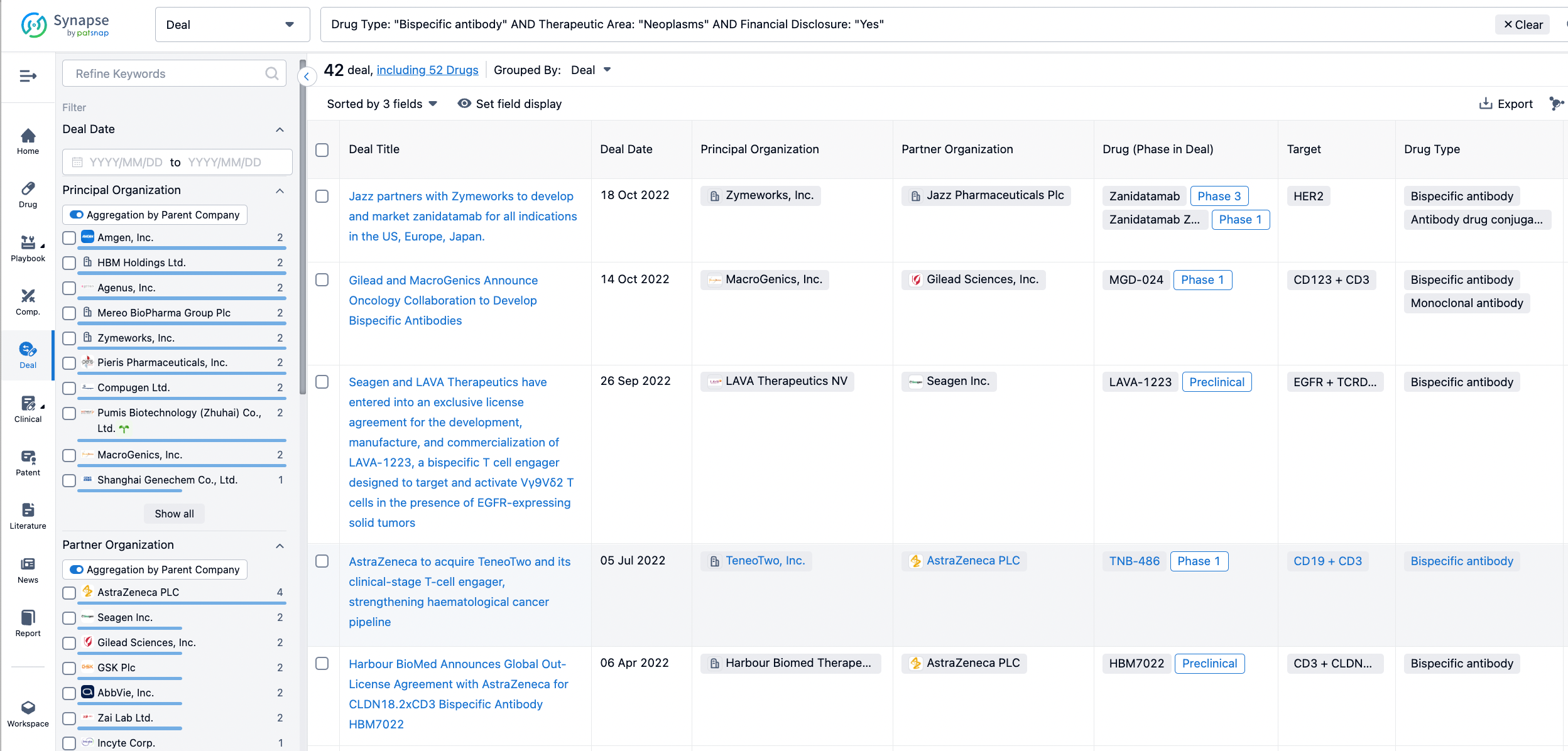
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
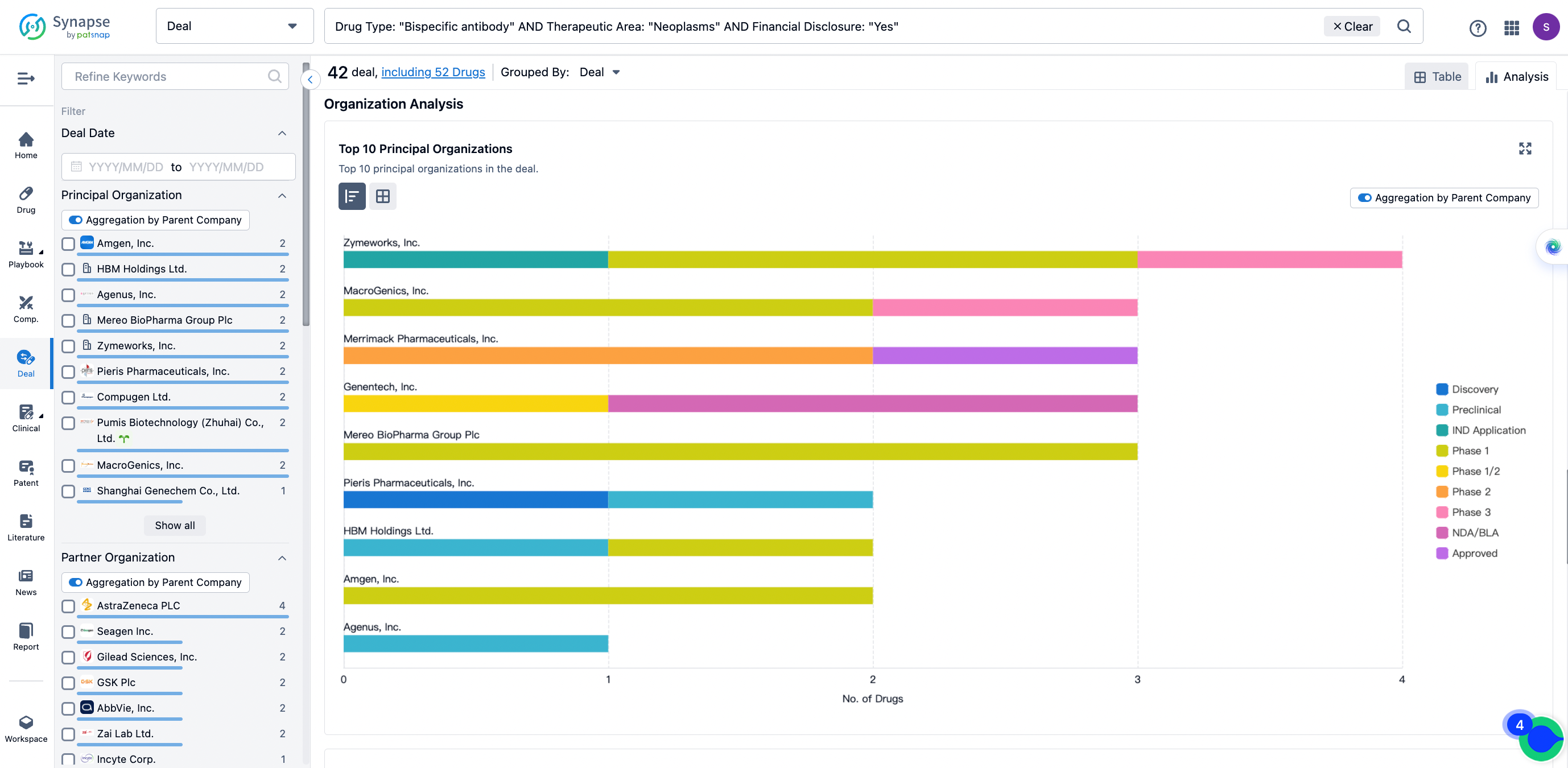
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
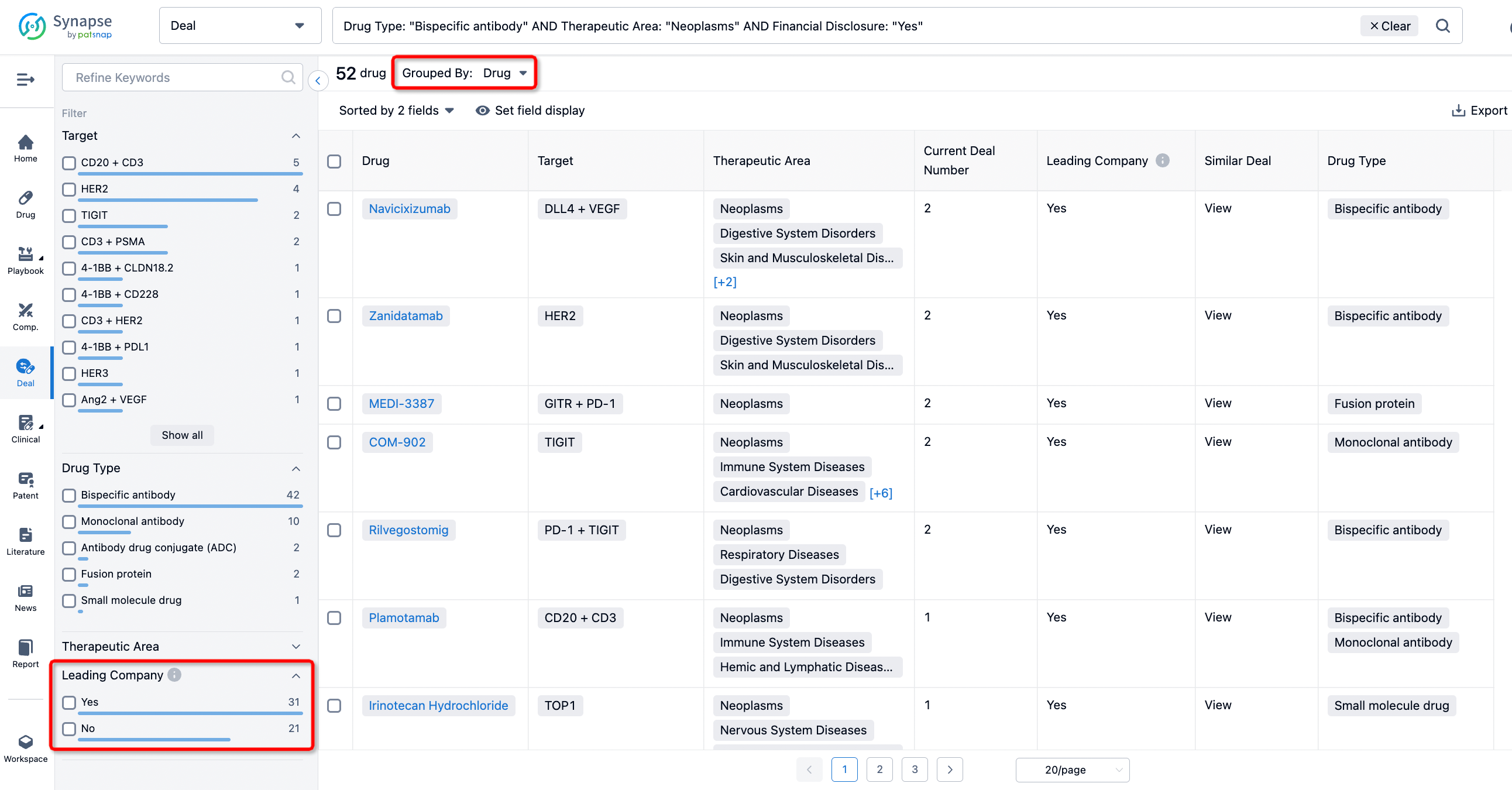
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!