Exploring treosulfan's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Treosulfan's R&D Progress
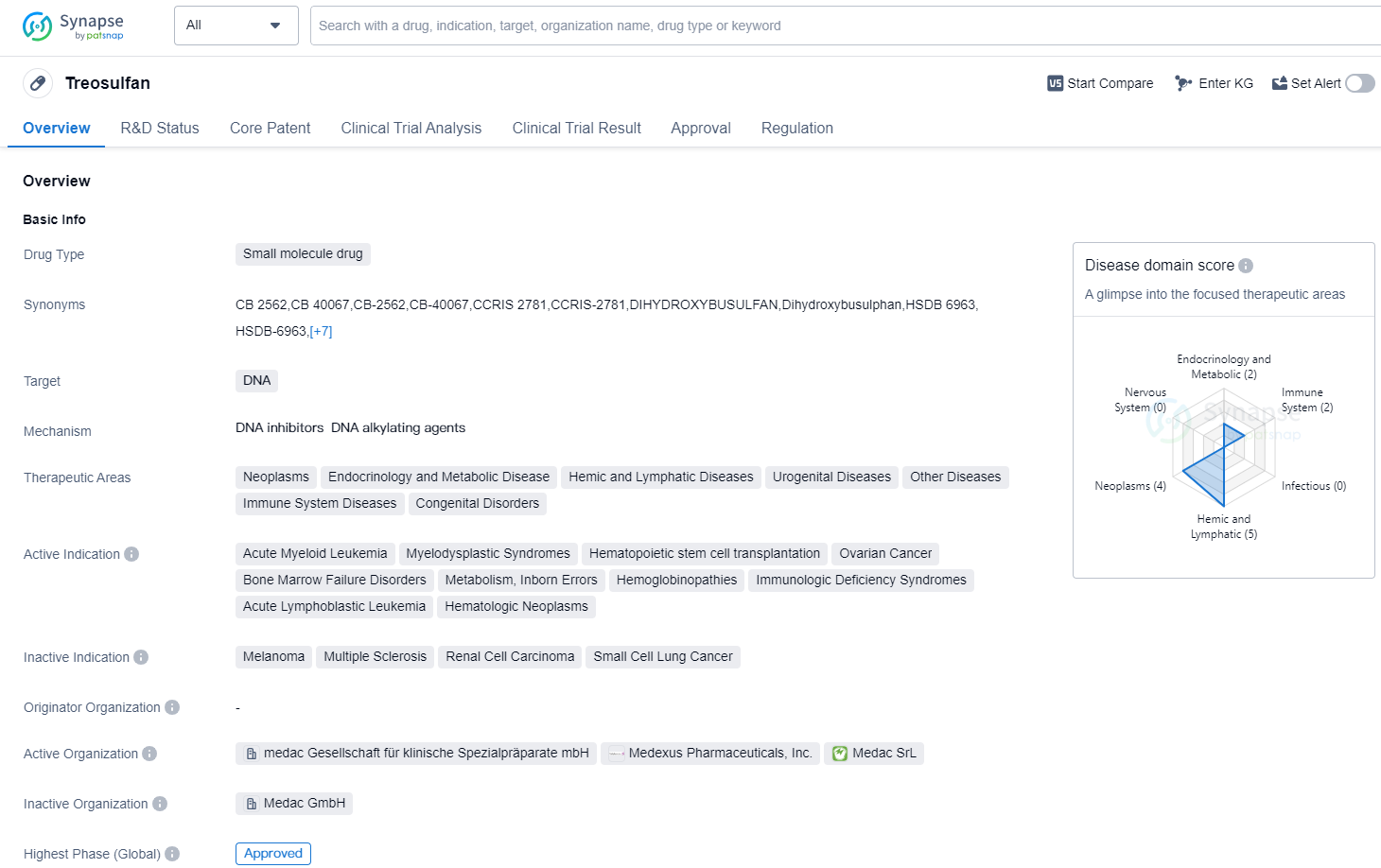
Treosulfan is a small molecule drug that targets DNA and has been approved for use in various therapeutic areas. These areas include neoplasms (abnormal growth of cells), endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, immune system diseases, congenital disorders, and other diseases.
The drug has shown efficacy in treating several indications, including acute myeloid leukemia, myelodysplastic syndromes, hematopoietic stem cell transplantation, ovarian cancer, bone marrow failure disorders, metabolism inborn errors, hemoglobinopathies, immunologic deficiency syndromes, acute lymphoblastic leukemia, and hematologic neoplasms.
Treosulfan received its first approval in December 1982 in Germany. It is classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions. Orphan drugs often receive special regulatory incentives to encourage their development and availability to patients.
As a small molecule drug, Treosulfan is designed to interact with DNA, which plays a crucial role in the replication and functioning of cells. By targeting DNA, Treosulfan may disrupt the growth and division of cancer cells or correct abnormalities in genetic disorders.
The drug has undergone rigorous testing and evaluation to reach its approved status. It has successfully completed clinical trials and demonstrated its safety and efficacy in treating the specified indications.
Treosulfan's approval in 1982 suggests that it has been available for several decades, providing healthcare professionals with a long-standing treatment option for the specified therapeutic areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for treosulfan: DNA inhibitors DNA alkylating agents
DNA inhibitors are a class of compounds that interfere with the normal functioning of DNA, which is the genetic material present in all living organisms. These inhibitors can target various processes involved in DNA replication, transcription, and repair. By inhibiting these processes, DNA inhibitors can disrupt the growth and division of cells, making them useful in the treatment of cancer and other diseases.
DNA alkylating agents are a specific type of DNA inhibitor that work by adding alkyl groups to the DNA molecule. These alkyl groups can cause damage to the DNA structure and prevent proper replication and transcription. As a result, DNA alkylating agents can inhibit the growth of cancer cells by interfering with their ability to divide and multiply.
In the context of biomedicine, DNA inhibitors and DNA alkylating agents are important therapeutic tools for the treatment of cancer. They are often used in chemotherapy regimens to target rapidly dividing cancer cells and prevent their proliferation. These compounds can be administered orally, intravenously, or topically, depending on the specific drug and cancer type. It is worth noting that while DNA inhibitors can be effective in killing cancer cells, they can also affect normal cells, leading to side effects. Therefore, the use of these agents requires careful consideration and monitoring by healthcare professionals.
Drug Target R&D Trends for treosulfan
DNA, or deoxyribonucleic acid, is a fundamental molecule that carries the genetic instructions for the development, functioning, and reproduction of all living organisms, including humans. It is present in the nucleus of every cell and consists of a unique sequence of nucleotides. DNA plays a crucial role in determining an individual's traits, such as physical characteristics, susceptibility to diseases, and even behavior. It serves as a blueprint for the production of proteins, which are essential for the structure and function of cells. Understanding DNA enables scientists to unravel the mysteries of human biology, develop new therapies, and advance medical treatments.
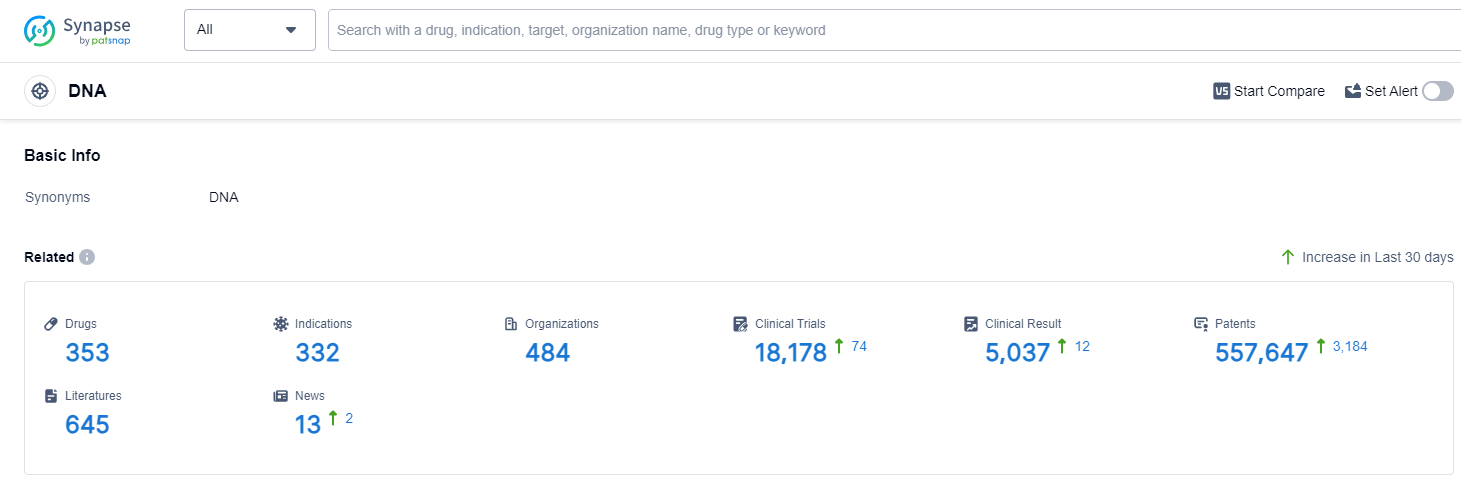
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 353 DNA drugs worldwide, from 484 organizations, covering 332 indications, and conducting 18178 clinical trials.
The analysis of the current competitive landscape and future development of the target DNA reveals that Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under the current target. Lymphoma, Ovarian Cancer, and Breast Cancer are the indications with the highest number of approved drugs. Small molecule drugs, Monoclonal antibodies, and Antibody drug conjugates are the drug types progressing most rapidly. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the current target DNA. The analysis suggests a competitive landscape with a focus on specific indications and drug types, with significant contributions from China in drug development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Treosulfan is a small molecule drug that targets DNA and has been approved for use in various therapeutic areas, including neoplasms, endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, immune system diseases, congenital disorders, and other diseases. It has shown efficacy in treating multiple indications, including acute myeloid leukemia, myelodysplastic syndromes, and ovarian cancer. Treosulfan received its first approval in Germany in 1982 and is classified as an orphan drug. Its approval and long-standing availability suggest its importance in the pharmaceutical industry.